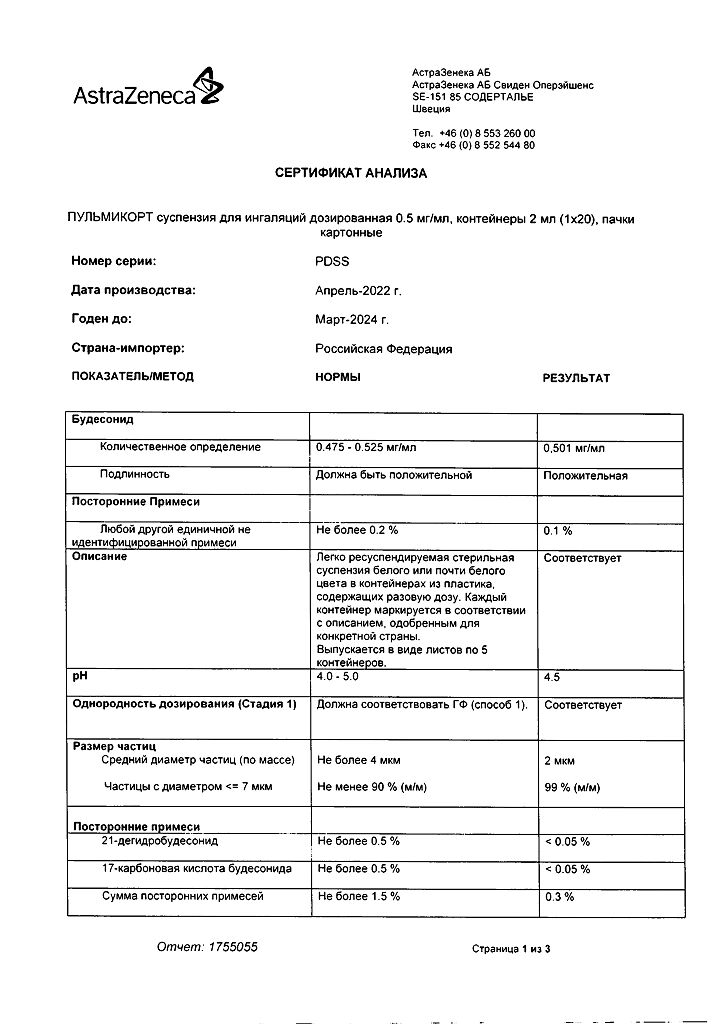
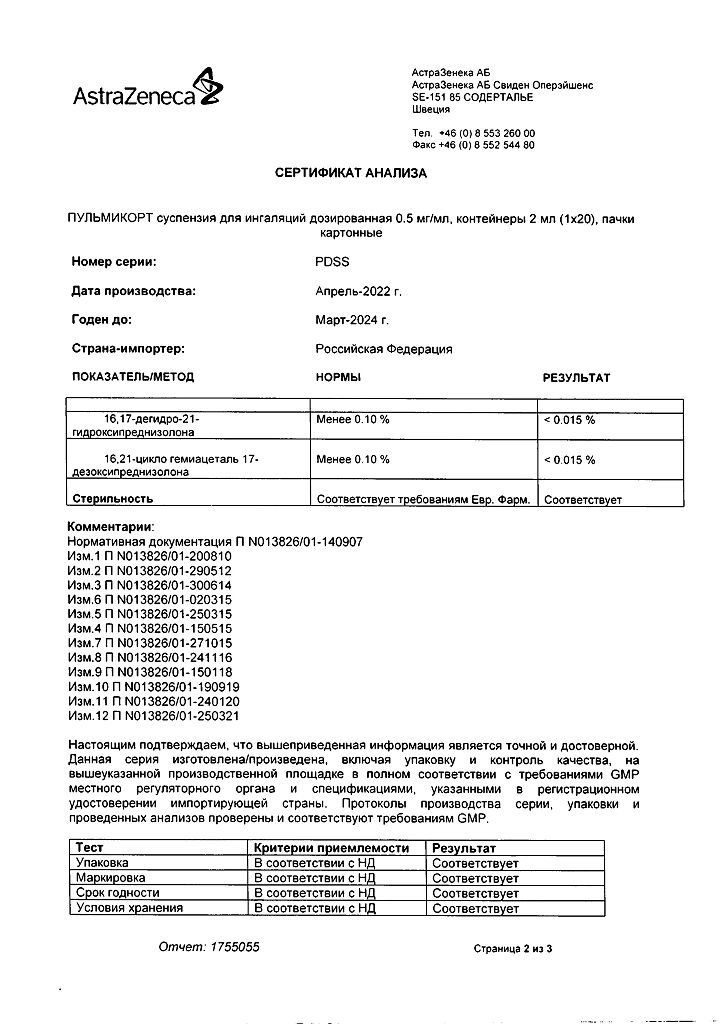
No products in the cart.
Pulmicort, 0.5 mg/ml 2 ml 20 pcs.
€24.85 €20.71
Description
Pharmacotherapeutic group Glucocorticosteroid for topical use. ATX code: R03BA02
Pharmacological Properties
Pharmacodynamics
Budesonide, an inhaled glucocorticosteroid, at recommended doses has an anti-inflammatory effect in the bronchi, reducing the severity of symptoms and the frequency of exacerbations of bronchial asthma with a lower frequency of side effects than with systemic glucocorticosteroids. It reduces the severity of bronchial mucosal edema, mucus production, sputum formation and airway hyperresponsiveness. It is well tolerated with long-term treatment, has no mineralocorticosteroid activity.
Dose-dependent effects on plasma and urinary cortisol levels during administration of Pulmicort® have been shown. At the recommended doses, the drug has significantly less effect on adrenal function than prednisolone at a dose of 10 mg, as shown in ACTH tests.
In bronchial asthma and the use of inhaled glucocorticosteroids, growth retardation may be noted. Studies with children and adolescents who received therapy with budesonide for a long time (up to 13 years) have shown that patients achieved the expected growth in adulthood.
Inhaled budesonide therapy is effective in preventing bronchial asthma of physical effort.
Clinical studies with exacerbations of chronic obstructive pulmonary disease (COPD.Several studies of nebulized budesonide at a dose of 4-8 mg/day have shown efficacy in the therapy of exacerbations of COPD.
Clinical Studies – Bronchial Asthma
The efficacy of Pulmicort® has been evaluated in numerous studies. The drug has been shown to be effective in the treatment of persistent bronchial asthma in both adults and children when used once or twice daily. Inhaled budesonide has also been shown to be effective in the therapy and prevention of exacerbations of bronchial asthma in children and adults.
Population of pediatric patients Clinical studies – false croup
A number of studies in children with false croup have compared Pulmicort® with placebo. Below are examples of representative studies evaluating the use of Pulmicort® for the treatment of children with false croup.
Effectiveness in children with mild to moderate false croup
A randomized, double-blind, placebo-controlled trial involving 87 children aged 7 months to 9 years hospitalized with a clinical diagnosis of false croup was conducted to determine whether Pulmicort improves the efficacy of Pulmicort®, a suspension, improved the assessment of false croup symptoms or shortened the duration of hospital treatment. Patients received Pulmicort®, suspension, at an initial dose of 2 mg or placebo, followed by Pulmicort® at a dose of 1 mg or placebo every 12 hours.
Pulmicort®, suspension, resulted in statistically significant reductions in the severity of false croup symptoms after 12 hours and 24 hours and 2 hours after administration in a subgroup of patients with a baseline symptom score above 3. The duration of inpatient treatment was also reduced by 33%.
Effectiveness in children with moderate to severe false croup
A randomized, double-blind, placebo-controlled trial compared the effectiveness of Pulmicort® and placebo in the treatment of false croup in 83 infants and children (ages 6 months to 8 years) hospitalized for false croup. Patients received Pulmicort®, suspension, 2 mg or placebo every 12 hours for up to 36 hours or until hospital discharge. An overall assessment of false croup symptoms was performed at 0, 2, 6, 12, 24, 36, and 48 hours after the initial dose was administered. After 2 hours, both the Pulmicort® and placebo groups showed a similar reduction in the severity of false croup symptoms, with no statistically significant difference between the groups. Six hours after the initial dose, there was a statistically significant reduction in the severity of false croup symptoms in the Pulmicort®, suspension group compared to the placebo group, and this improvement was similar after 12 hours and 24 hours.
Pharmacokinetics
.Absorption
Inhaled budesonide is rapidly absorbed. In adults, the systemic bioavailability of budesonide after inhalation of Pulmicort® via nebulizer is approximately 15% of the total dose administered and approximately 40-70% of the dose delivered.
The maximum plasma concentration is reached 30 minutes after initiation of inhalation.
Metabolism and distribution
The binding to plasma proteins averages 90%. The volume of distribution of budesonide is approximately 3 L/kg. After absorption budesonide undergoes intensive (over 90%) biotransformation in the liver to form metabolites with low glucocorticosteroid activity. The glucocorticosteroid activity of the main metabolites 6b-hydroxybudesonide and 16a-hydroxyprednisolone is less than 1% of the glucocorticosteroid activity of budesonide.
Elimation
Budesonide is metabolized mainly with participation of CYP3A4 enzyme. Metabolites are excreted unchanged in the urine or in conjugated form. Budesonide has high systemic clearance (about 1.2 l/min). Pharmacokinetics of budesonide is proportional to the administered dose of the drug.
The pharmacokinetics of budesonide in patients with impaired renal function has not been studied. Exposure to budesonide may be increased in patients with liver disease.
Indications
Indications
• Bronchial asthma requiring therapy with glucocorticosteroids for:
– maintenance therapy
– exacerbations, when the use of budesonide in the form of an inhalation suspension is justified.
• Chronic obstructive pulmonary disease (COPD) for:
– maintenance therapy
– exacerbations, when the use of budesonide in the form of an inhalation suspension as an alternative to systemic glucocorticosteroids is justified.
• Stenosing laryngotracheitis (false croup).
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group Glucocorticosteroid for local use.
ATX code: R03BA02
PHARMACOLOGICAL PROPERTIES
Special instructions
Special instructions
Budesonide is not intended for rapid relief of acute asthma attacks that require the use of short-acting inhaled bronchodilators.
In case of exacerbation, you can increase the dose of Pulmicort® or prescribe short-term additional therapy.
As with other inhaled therapy, paradoxical bronchospasm may occur with an immediate increase in wheezing after using Pulmicort®. In this case, inhaled budesonide therapy should be stopped immediately, the patient’s condition assessed and, if necessary, alternative therapy prescribed.
Fungal infections of the oral cavity can occur during the use of inhaled glucocorticosteroids. If such an infection develops, it may be necessary to use appropriate antifungal agents, and in some patients, discontinuation of inhaled corticosteroids. To reduce the risk of developing fungal infections of the oropharynx, the patient should be instructed to thoroughly rinse their mouth with water after each inhalation of the drug.
Co-administration of budesonide with ketoconazole, itraconazole or other strong CYP3A4 inhibitors should be avoided. If budesonide and ketoconazole or other strong CYP3A4 inhibitors have been prescribed, the time between doses should be increased to the maximum possible.
Due to the possible risk of weakening adrenal function, special attention should be paid to patients who are switching from oral glucocorticosteroids to taking Pulmicort®. Also, special attention should be paid to patients taking high doses of glucocorticosteroids, or who have been receiving the highest recommended doses of inhaled glucocorticosteroids for a long time. In stressful situations, these patients may exhibit signs and symptoms of adrenal insufficiency. In case of stress or in cases of surgical intervention, additional therapy with systemic glucocorticosteroids is recommended.
Particular attention should be paid to patients who are transferred from systemic to inhaled glucocorticosteroids (Pulmicort®) or in cases where a violation of the pituitary-adrenal function can be expected. In such patients, the dose of systemic glucocorticosteroids should be reduced with extreme caution and hypothalamic-pituitary-adrenal function should be monitored. Patients may also require the addition of oral corticosteroids during stressful situations such as trauma, surgery, etc.
When switching from oral glucocorticosteroids to Pulmicort®, patients may experience previously observed symptoms, such as muscle pain or joint pain. In such cases, a temporary increase in the dose of oral corticosteroids may be necessary. In rare cases, symptoms such as fatigue, headache, nausea and vomiting may occur, indicating systemic glucocorticosteroid deficiency.
Replacing oral glucocorticosteroids with inhaled ones sometimes leads to the development of concomitant allergies, for example, rhinitis and eczema, which were previously treated with systemic drugs.
In children and adolescents receiving treatment with glucocorticosteroids (regardless of the method of delivery) for an extended period, it is recommended to regularly monitor growth parameters. If growth is delayed, therapy should be reconsidered to reduce the dose of inhaled corticosteroid, if possible, to the lowest dose that maintains effective control of bronchial asthma. It is necessary to carefully evaluate the balance between the benefits of glucocorticosteroid therapy and the possible risk of growth retardation. In addition, it is recommended that the patient be referred to a pediatric pulmonologist.
The use of budesonide at a dose of up to 400 mcg per day in children over 3 years of age did not lead to systemic effects. Biochemical signs of a systemic effect of the drug may occur when taking the drug at a dose of 400 to 800 mcg per day. When the dose exceeds 800 mcg per day, systemic effects of the drug are common.
Systemic effects may occur with the use of any inhaled glucocorticosteroids, especially when used in high doses over a long period of time. The likelihood of developing such symptoms is significantly less with inhaled glucocorticosteroid therapy than with oral glucocorticosteroids.
Possible systemic effects include Cushing’s syndrome, Cushingoid features, adrenal suppression, growth retardation in children and adolescents, decreased bone mineral density, cataracts, glaucoma, psychological symptoms and behavioral disturbances including psychomotor hyperactivity, sleep disturbances, anxiety, depression and aggression, especially in children (see Side Effects section). The dose of inhaled glucocorticosteroid should be the lowest at which effective disease control is maintained.
Impaired liver function may affect the elimination of corticosteroids, causing a decrease in the rate of elimination and an increase in systemic exposure. Patients should be warned about possible systemic adverse reactions.
Clinical studies and meta-analyses have shown that the use of inhaled corticosteroids during maintenance treatment of COPD may lead to an increased risk of pneumonia. Clinicians should be aware of the possibility of pneumonia in patients with COPD, since the clinical signs of pneumonia and exacerbation of the disease often overlap.
Visual impairment may occur with systemic and local use of glucocorticosteroids. If a patient develops symptoms such as blurred vision or other visual disturbances, consider referring the patient to an ophthalmologist to evaluate possible causes, which may include cataracts, glaucoma, or rare diseases such as central serous retinopathy (CSR) that have been reported after systemic and topical corticosteroid use.
INFLUENCE ON THE ABILITY TO DRIVE A CAR OR OTHER MECHANISMS
Pulmicort® does not affect the ability to drive a car or use other machinery.
Active ingredient
Active ingredient
Budesonide
Composition
Composition
1 ml of suspension contains:
Active ingredient: budesonide (micronized budesonide) 0.25 mg or 0.5 mg.
Auxiliary ingredients: sodium chloride 8.5 mg, sodium citrate 0.5 mg, disodium edetate (sodium salt of ethylenediaminetetraacetic acid (disubstituted) (disodium salt EDTA)) 0.1 mg, polysorbate 80 0.2 mg, citric acid (anhydrous) 0.28 mg, purified water to 1 ml.
Pregnancy
Pregnancy
Pregnancy: observation of pregnant women taking budesonide did not reveal developmental abnormalities in the fetus, however, the risk of their development cannot be completely excluded, therefore, during pregnancy, due to the possibility of worsening the course of bronchial asthma, the minimum effective dose of budesonide should be used.
Lactation: Budesonide passes into breast milk, however, when using the drug Pulmicort® in therapeutic doses, no effect on the child was noted. Pulmicort® can be used during breastfeeding.
Contraindications
Contraindications
• Hypersensitivity to budesonide.
• Children up to 6 months.
With caution: pulmonary tuberculosis (active or inactive form), fungal, viral or bacterial infections of the respiratory system, cirrhosis of the liver, pregnancy, breastfeeding.
Side Effects
Side Effects
The frequency of occurrence of undesirable effects is presented as follows:
Often (> 1/100, 1/1000, 1/10000, < 1/1000); Very rare (<1/10000), including isolated reports.
Up to 10% of patients taking the drug may experience the following side effects:
Often
Respiratory tract:
Oropharyngeal candidiasis, mild irritation of the throat mucosa, cough, hoarseness, dry mouth, pneumonia (in patients
with COPD).
Rarely
General:
Leather:
Respiratory tract: Central nervous system:
Immune system:
Gastrointestinal tract:
Angioedema.
The appearance of bruises on the skin.
Bronchospasm. Nervousness, excitability,
depression, behavioral disorders.
Immediate and hypersensitivity reactions
delayed type, including rash, contact dermatitis, urticaria, angioedema, bronchospasm and anaphylactic reaction.
Nausea.
Very rarely
Laboratory indicators:
Sense organs:
Decrease in bone mineral density (systemic effect).
Cataract, glaucoma
(systemic action).
Taking into account the risk of developing oropharyngeal candidiasis, the patient should thoroughly rinse his mouth with water after each inhalation of the drug.
In rare cases, symptoms caused by systemic corticosteroids may occur, including adrenal hypofunction and growth retardation in children. The severity of these symptoms likely depends on the dose of the drug, duration of therapy, concomitant or previous therapy with glucocorticosteroids, and individual sensitivity.
There have been cases of facial skin irritation when using a nebulizer with a mask. To prevent irritation, your face should be washed with water after using the mask.
Interaction
Interaction
No interaction of budesonide with other drugs used in the treatment of bronchial asthma has been observed.
Ketoconazole (200 mg once daily) increases plasma concentrations of oral budesonide (3 mg once daily) by an average of 6-fold when administered together. When taking ketoconazole 12 hours after taking budesonide, the concentration of the latter in the blood plasma increased by an average of 3 times. There is no information on such an interaction when taking budesonide in the form of inhalation, but it is assumed that in this case an increase in the concentration of budesonide in the blood plasma should be expected. If it is necessary to take ketoconazole and budesonide, the time between doses of the drugs should be increased to the maximum possible. A dose reduction of budesonide should also be considered. Another potential inhibitor of CYP3A4, such as itraconazole, also significantly increases plasma concentrations of budesonide.
Pre-inhalation of beta-adrenergic stimulants dilates the bronchi, improves the entry of budesonide into the respiratory tract and enhances its therapeutic effect.
Phenobarbital, phenytoin, rifampicin reduce the effectiveness (induction of microsomal oxidation enzymes) of budesonide.
Methandrostenolone, estrogens enhance the effect of budesonide.
Overdose
Overdose
In case of acute overdose, no clinical manifestations occur. With long-term use of the drug in doses significantly higher than recommended, a systemic glucocorticosteroid effect may develop in the form of hypercortisolism and suppression of adrenal function.
Storage conditions
Storage conditions
At a temperature not exceeding 30 C. Do not freeze. Keep out of the reach of children.
The drug in containers must be used within 3 months after opening the envelope. An opened container must be used within 12 hours. Containers should be stored in an envelope to protect them from light.
Shelf life
Shelf life
2 years. Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
AstraZeneca AB, Sweden
Additional information
| Shelf life | 2 years. Do not use after the expiration date stated on the package. |
|---|---|
| Conditions of storage | At a temperature not exceeding 30 C. Do not freeze. Keep out of reach of children. The drug in containers should be used within 3 months after opening the envelope. An open container should be used within 12 hours. Containers should be stored in an envelope to protect them from light. |
| Manufacturer | AstraZeneca AB, Sweden |
| Medication form | metered suspension for inhalations |
| Brand | AstraZeneca AB |
Other forms…
Related products
Buy Pulmicort, 0.5 mg/ml 2 ml 20 pcs. with delivery to USA, UK, Europe and over 120 other countries.