No products in the cart.
Description
Protafan HM penfill has hypoglycemic action.
It interacts with the specific receptor of the plasma membrane and penetrates into the cell, where it activates phosphorylation of cellular proteins, stimulates glycogen synthetase, pyruvate dehydrogenase, hexokinase, inhibits lipase of fat tissue and lipoproteinlipase. In complex with specific receptor it facilitates glucose penetration into cells, enhances its uptake by tissues and promotes conversion into glycogen. It increases the stock of glycogen in the muscles and stimulates the synthesis of peptides.
The effect develops 1.5 hours after p/u injection, reaches a maximum after 4-12 hours and lasts 24 hours. Protafan NM Penfill at insulin-dependent diabetes is used as basal insulin in combination with short-acting insulin, at insulin-independent – both for monotherapy and in combination with fast-acting insulins.
Indications
Indications
Diabetes mellitus type I, diabetes mellitus type II (with resistance to sulfonylurea derivatives, intercurrent diseases, operations and in the postoperative period, during pregnancy).
Pharmacological effect
Pharmacological effect
Protafan HM penfill has a hypoglycemic effect.
It interacts with a specific plasma membrane receptor and penetrates the cell, where it activates the phosphorylation of cellular proteins, stimulates glycogen synthetase, pyruvate dehydrogenase, hexokinase, inhibits adipose tissue lipase and lipoprotein lipase. In combination with a specific receptor, it facilitates the penetration of glucose into cells, enhances its absorption by tissues and promotes conversion into glycogen. Increases glycogen reserves in muscles, stimulates peptide synthesis.
The effect develops 1.5 hours after subcutaneous administration, reaches a maximum after 4–12 hours and lasts 24 hours. Protafan NM Penfill in insulin-dependent diabetes mellitus is used as basal insulin in combination with short-acting insulin, in non-insulin-dependent diabetes mellitus – both for monotherapy and in combination with fast-acting insulins.
Active ingredient
Active ingredient
Insulin-isophane human genetically engineered
Composition
Composition
Active ingredient:
insulin isophane (human genetically engineered);
Excipients:
zinc chloride;
glycerin (glycerol);
metacresol;
phenol;
sodium hydrogen phosphate dihydrate;
protamine sulfate;
sodium hydroxide and/or hydrochloric acid (to adjust pH);
water for injections
Contraindications
Contraindications
Hypoglycemia, insulinoma.
Side Effects
Side Effects
Hypoglycemic conditions, allergic reactions, lipodystrophy (with long-term use).
Interaction
Interaction
The hypoglycemic effect is enhanced by acetylsalicylic acid, alcohol, alpha- and beta-blockers, amphetamine, anabolic steroids, clofibrate, cyclophosphamide, fenfluramine, fluoxetine, ifosfamide, MAO inhibitors, methyldopa, tetracyclines, tritoqualine, triphosphamide, weakened by chlorprothixene, diazoxide, diuretics (especially thiazides), glucocorticoids, heparin, hormonal contraceptives, isoniazid, lithium carbonate, nicotinic acid, phenothiazines, sympathomimetics, tricyclic antidepressants.
Overdose
Overdose
Symptoms: development of hypoglycemia (cold sweat, palpitations, tremors, hunger, agitation, irritability, pallor, headache, drowsiness, uncertainty of movements, speech and vision disturbances, depression). Severe hypoglycemia can lead to temporary or permanent impairment of brain function, coma, and death.
Treatment: sugar or glucose solution orally (if the patient is conscious), subcutaneous, intramuscular or intravenous – glucagon or intravenous – glucose.
Storage conditions
Storage conditions
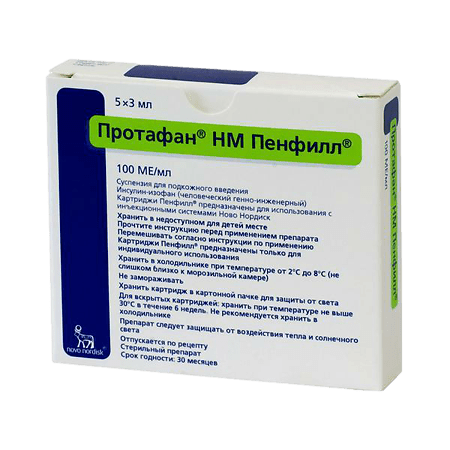
In a place protected from light, at a temperature of 2–8 °C (do not freeze). Store the used bottle at room temperature not exceeding 30 °C for 6 weeks. Protect from heat and sunlight.
Shelf life
Shelf life
30 months
Manufacturer
Manufacturer
Novo Nordisk A/S, Denmark
Additional information
| Shelf life | 30 months |
|---|---|
| Conditions of storage | Store in a light-protected place at 2-8 °C (do not freeze). Store used vial at room temperature not exceeding 30 °C for 6 weeks. Protect from heat and sunlight. |
| Manufacturer | Novo Nordisk A/S, Denmark |
| Medication form | suspension |
| Brand | Novo Nordisk A/S |
Related products
Buy Protafan HM Penfill, 100 me/ml suspension 3 ml 5 pcs with delivery to USA, UK, Europe and over 120 other countries.