No products in the cart.
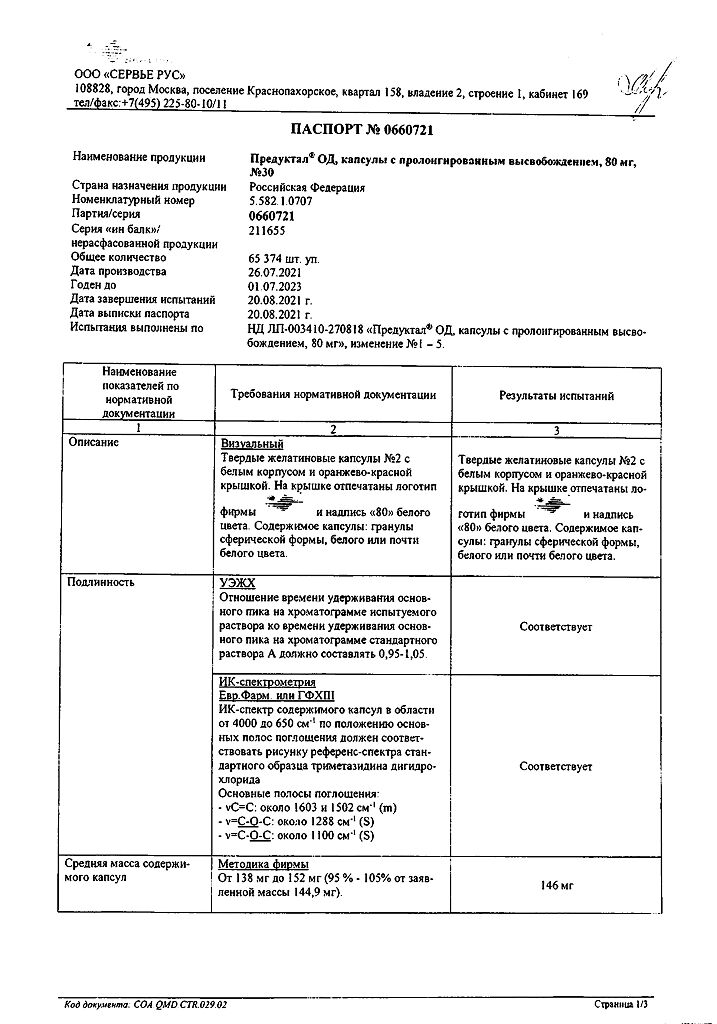
Preduktal OD, 80 mg 30 pcs
€24.71 €21.41
Description
Trimetazidine prevents a decrease in intracellular adenosine triphosphate (ATP) concentration by preserving the energy metabolism of cells under hypoxia. Thus, the drug ensures normal functioning of membrane ion channels, transmembrane transport of potassium and sodium ions and preservation of cellular homeostasis.
Trimetazidine inhibits fatty acid oxidation through selective inhibition of the enzyme 3-ketoacyl-CoA-thiolase (3-CAT) mitochondrial long-chain fatty acid isoform, which leads to increased glucose oxidation and accelerated glucose-oxidized glycolysis, resulting in myocardial protection from ischemia. The switch of energy metabolism from fatty acid oxidation to glucose oxidation underlies the pharmacological properties of trimetazidine.
Pharmacodynamic properties
– Supports energy metabolism of the heart and neurosensory tissues during ischemia;
– reduces the severity of intracellular acidosis and changes in transmembrane ion flow occurring during ischemia;
– decreases the level of migration and infiltration of polynuclear neutrophils in ischemic and reperfused heart tissues;
– reduces the size of myocardial damage;
– has no direct effect on hemodynamic parameters.
In patients with angina pectoris, trimetazidine increases coronary reserve, thereby delaying the onset of exercise-induced ischemia from day 15 of therapy; it limits exercise-induced BP fluctuations without significant changes in HR; significantly reduces the frequency of angina attacks and the need for short-acting nitroglycerin; improves left ventricular contractile function in patients with ischemic dysfunction.
The results of clinical studies have confirmed efficacy and safety of trimetazidine in patients with stable angina pectoris both in monotherapy and in combination therapy when other antianginal drugs have insufficient effect.
In a study involving 426 patients with stable angina pectoris, the addition of trimetazidine (60 mg/day) to therapy with metoprolol 100 mg/day (50 mg 2 times/day) for 12 weeks statistically significantly improved exercise test results and clinical symptoms compared with placebo: total exercise test duration was +20.1 s, p=0.023, total time to load performance +0.54 METs, p=0.001, time to ST segment depression of 1 mm +33.4 s, p=0.003, time to angina attack development +33.9 s, p< 0.001, number of angina attacks per week -0.73, p=0.014 and short-acting nitrate consumption per week -0.63, p=0.032, no hemodynamic changes.
In a study involving 223 patients with stable angina pectoris, the addition of trimetazidine at a dose of 35 mg (2 times/day) to therapy with atenolol at 50 mg (once/day) for 8 weeks resulted in a 1-mm increase in time to development of ischemic ST-segment depression (+34.4 s, p=0.03) on loading tests in a subgroup of patients (n=173), compared with placebo, 12 h after drug administration. This difference was also shown for the timing of angina attacks (p=0.049). No significant differences were found between groups for other secondary endpoints (total exercise test duration, total exercise time, and clinical endpoints).
In a study involving 1962 patients with stable angina pectoris, trimetazidine at two doses (70 mg/day and 140 mg/day) compared with placebo was added to therapy with atenolol 50 mg/day. In the general population, including both asymptomatic and symptomatic patients with angina, trimetazidine showed no advantage in ergometric (total duration of exercise tests, time to onset of ischemic ST-segment depression by 1 mm and time to angina attack) and clinical endpoints. However, in a retrospective analysis in a subgroup of patients with symptomatic angina (n=1574), trimetazidine (140 mg) significantly improved overall load test time (+23.8 s vs. +13.1 s for placebo; p=0.001) and time to angina attack development (+46.3 s vs. +32.5 for placebo; p=0.005).
Indications
Indications
Long-term therapy of coronary artery disease: prevention of attacks of stable angina as part of mono- or combination therapy.
Pharmacological effect
Pharmacological effect
Trimetazidine prevents a decrease in intracellular adenosine triphosphate (ATP) concentration by maintaining the energy metabolism of cells in a state of hypoxia. Thus, the drug ensures the normal functioning of membrane ion channels, transmembrane transport of potassium and sodium ions and the preservation of cellular homeostasis.
Trimetazidine inhibits the oxidation of fatty acids due to the selective inhibition of the enzyme 3-ketoacyl-CoA thiolase (3-CAT) of the mitochondrial long-chain isoform of fatty acids, which leads to increased oxidation of glucose and acceleration of glycolysis with oxidation of glucose, which determines the protection of the myocardium from ischemia. The switch of energy metabolism from fatty acid oxidation to glucose oxidation underlies the pharmacological properties of trimetazidine.
Pharmacodynamic properties
– supports the energy metabolism of the heart and neurosensory tissues during ischemia;
– reduces the severity of intracellular acidosis and changes in the transmembrane ion flow that occur during ischemia;
– reduces the level of migration and infiltration of polynuclear neutrophils in ischemic and reperfused heart tissues;
– reduces the size of myocardial damage;
– does not have a direct effect on hemodynamic parameters.
In patients with angina pectoris, trimetazidine increases coronary reserve, thereby slowing the onset of exercise-induced ischemia, starting from the 15th day of therapy; limits fluctuations in blood pressure caused by physical activity without significant changes in heart rate; significantly reduces the frequency of angina attacks and the need for short-acting nitroglycerin; improves contractile function of the left ventricle in patients with ischemic dysfunction.
The results of clinical studies have confirmed the effectiveness and safety of trimetazidine in patients with stable angina, both in monotherapy and as part of combination therapy when the effect of other antianginal drugs is insufficient.
In a study of 426 patients with stable angina, the addition of trimetazidine (60 mg/day) to metoprolol 100 mg/day (50 mg twice daily) for 12 weeks statistically significantly improved exercise test scores and clinical symptoms compared with placebo: total exercise test duration was +20.1 s, p = 0.023, total exercise time +0.54 METs, p=0.001, time to development of ST segment depression by 1 mm +33.4 s, p=0.003, time to development of angina attack +33.9 s, p<0.001, number of angina attacks per week -0.73, p=0.014 and consumption of short-acting nitrates per week -0.63, p=0.032, without hemodynamic changes.
In a study of 223 patients with stable angina, the addition of trimetazidine 35 mg twice daily to atenolol 50 mg once daily for 8 weeks resulted in a 1 mm increase in time to ischemic ST segment depression (+34.4 s, p = 0.03) during exercise testing in a subgroup of patients (n = 173) compared with placebo. 12 hours after taking the drug. This difference was also shown for the time of development of angina attacks (p=0.049). There were no significant differences between groups for other secondary endpoints (total exercise test duration, total exercise time, and clinical endpoints).
In a study of 1962 patients with stable angina, trimetazidine at two doses (70 mg/day and 140 mg/day) was added to atenolol 50 mg/day versus placebo. In the general population, including patients with both asymptomatic and symptomatic angina, trimetazidine did not demonstrate benefit on ergometric (total duration of exercise tests, time to onset of ischemic ST-segment depression of 1 mm and time to onset of angina) and clinical endpoints. However, in a post-hoc analysis of a subgroup of patients with symptomatic angina (n=1574), trimetazidine (140 mg) significantly improved total exercise test time (+23.8 s versus +13.1 s for placebo; P=0.001) and time to onset of angina (+46.3 s versus +32.5 for placebo; P=0.005).
Special instructions
Special instructions
Preductal® OD is not intended for the relief of angina attacks and is not indicated for the initial course of treatment of unstable angina or myocardial infarction in the prehospital stage or in the first days of hospitalization.
If an attack of angina occurs, treatment (drug therapy or revascularization procedure) should be reviewed and adapted. Preductal® OD can cause or worsen symptoms of parkinsonism (tremor, akinesia, increased tone), so patients should be regularly monitored, especially the elderly. In doubtful cases, the patient should be referred to a neurologist for appropriate examination.
If movement disorders appear, such as symptoms of parkinsonism, restless legs syndrome, tremor, or “wobbly” gait, Preductal® OD should be permanently discontinued. Such cases are rare and symptoms usually resolve after discontinuation of therapy: in most patients, within 4 months after discontinuation of the drug. If symptoms of parkinsonism persist more than 4 months after discontinuation of the drug, you should consult a neurologist.
Cases of falls associated with instability in the Romberg position and “wobbly” gait or a pronounced decrease in blood pressure may occur, especially in patients taking antihypertensive drugs (see section “Side Effects”).
Preductal® OD should be prescribed with caution to patients in whom increased exposure may occur:
– in case of moderate renal failure (see sections “Pharmacological action” and “Dosage regimen”);
– in elderly patients over 75 years of age (see section “Dosage regimen”).
The drug contains sucrose, so the drug is not recommended for patients with fructose intolerance, glucose-galactose malabsorption syndrome and sucrase-isomaltase deficiency.
Impact on the ability to drive vehicles and operate machinery
During clinical studies, no effect of trimetazidine on hemodynamic parameters was revealed, however, during post-registration use, cases of dizziness and drowsiness were observed (see section “Side effects”). These symptoms can affect the ability to drive vehicles and perform work that requires increased speed of physical and mental reactions.
Active ingredient
Active ingredient
Trimetazidine
Composition
Composition
1 caps. – trimetazidine dihydrochloride (in granules) 80 mg.
1 extended-release hard capsule contains:
film-coated granules with a layer of trimetazidine dihydrochloride: 144.85 mg.
Excipients:
sugar spheres (710-850 microns) – 36.68 mg,
hypromellose – 6.4 mg.
Composition of the film shell of granules:
ethylcellulose – 8 mg,
tributylacetyl citrate – 1.2 mg,
talc – 12 mg.
Composition of the mixture for dusting granules:
talc – 0.43 mg,
magnesium stearate – 0.14 mg.
Contraindications
Contraindications
– severe renal failure (creatinine clearance less than 30 ml/min);
– Parkinson’s disease, symptoms of parkinsonism, tremor, restless legs syndrome and other associated movement disorders;
– fructose/sucrose intolerance, glucose-galactose malabsorption syndrome, sucrase/isomaltase deficiency and other enzymopathies associated with intolerance to sucrose, which is part of the drug;
– age under 18 years (due to lack of sufficient clinical data);
– hypersensitivity to any of the components of the drug.
The drug should be prescribed with caution to patients with severe liver failure (from 10 to 15 points on the Child-Pugh scale), moderate renal failure (creatinine clearance 30-60 ml/min), and patients over 75 years of age (see sections “Dosage regimen” and “Special instructions”).
Side Effects
Side Effects
Adverse reactions, defined as adverse events at least possibly related to treatment with trimetazidine, are given in the following gradation: very often (≥1/10); often (≥1/100, <1/10); uncommon (≥1/1000, <1/100); rare (≥1/10,000, <1/1000); very rare (< 1/10,000), unspecified frequency (frequency cannot be calculated from available data).
From the side of the central nervous system: often – dizziness, headache; unspecified frequency – symptoms of parkinsonism (tremor, akinesia, increased tone), “unsteadiness” of gait, restless legs syndrome, other associated motor disorders, usually reversible after cessation of therapy. Sleep disorders (insomnia, drowsiness).
From the cardiovascular system: rarely – a feeling of palpitations, extrasystole, tachycardia, a marked decrease in blood pressure, orthostatic hypotension, which may be accompanied by general weakness, dizziness or loss of balance, especially with the simultaneous use of antihypertensive drugs, “flushes” of blood to the skin of the face.
From the digestive system: often – abdominal pain, diarrhea, dyspepsia, nausea, vomiting; unspecified frequency – constipation.
From the liver and biliary tract: unspecified frequency – hepatitis.
From the hematopoietic system: unspecified frequency – agranulocytosis, thrombocytopenia, thrombocytopenic purpura.
From the skin and subcutaneous fat: often – skin rash, itching, urticaria; unspecified frequency – Quincke’s edema, acute generalized exanthematous pustulosis.
General disorders: often – asthenia.
Interaction
Interaction
Not observed. The patient must inform the doctor about all medications taken.
Overdose
Overdose
There is only very limited information on trimetazidine overdose.
Treatment: in case of overdose, symptomatic therapy should be carried out.
Manufacturer
Manufacturer
Servier Rus LLC, Russia
Additional information
| Manufacturer | Servier Rus LLC, Russia |
|---|---|
| Medication form | modified-release capsules |
| Brand | Servier Rus LLC |
Related products
Buy Preduktal OD, 80 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.