No products in the cart.
Potassium chloride, 40 mg/ml concentrate 10 ml 10 pcs
€4.22 €3.75
EAN: 4680013242626
SKU: 287692
Categories: Anesthesia and resuscitation, Anesthesia solutions, Medicine
Description
Pharmacotherapeutic group:
Potassium drug.
ATX code: A121BA01
Pharmacological action:
The K+ drug, restores water-electrolyte balance. It has negative chrono- and batmotropic effects, in high doses it has negative in- and dro-motropic and moderate diuritic effects. In low doses K+ dilates coronary vessels, in high doses it narrows them. It participates in the process of conducting nerve impulses. When administered intravenously it increases the release of epinephrine by the adrenal glands.
Activates many cytoplasmic enzymes, participates in maintaining intracellular osmotic pressure, in protein-synthetic reactions and transport of amino acids. It improves skeletal muscle contraction in muscular dystrophy and myasthenia. Increased K+ concentration reduces the risk of toxic effects of cardiac glycosides.
Indications
Indications
Hyperkalemia, complete atrioventricular block, adrenal insufficiency, chronic renal failure, concomitant therapy with potassium-sparing diuretics, metabolic disorders (acidosis, hypovolemia with hyponatremia), pregnancy, lactation, age under 18 years (efficacy and safety have not been established).
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group:
potassium preparation.
ATX code: A121BA01
Pharmacological action:
The drug K+ restores water-electrolyte balance. It has a negative chrono- and batmotronic effect, in high doses – a negative ino- and dromotropic effect, as well as a moderate diuretic effect. In small doses, K+ dilates the coronary vessels, in large doses it constricts. Participates in the process of conducting nerve impulses. When administered intravenously, it increases the secretion of epinephrine by the adrenal glands.
Activates many cytoplasmic enzymes, participates in maintaining intracellular osmotic pressure, in protein synthetic reactions and amino acid transport. Improves skeletal muscle contraction in muscular dystrophy, myasthenia gravis. Increasing K+ concentration reduces the risk of toxic effects of cardiac glycosides.
Special instructions
Special instructions
During the treatment period, it is necessary to monitor the K+ content in the blood serum, ECG, and when treating hypokalemia, control the acid-base state.
Active ingredient
Active ingredient
Potassium chloride
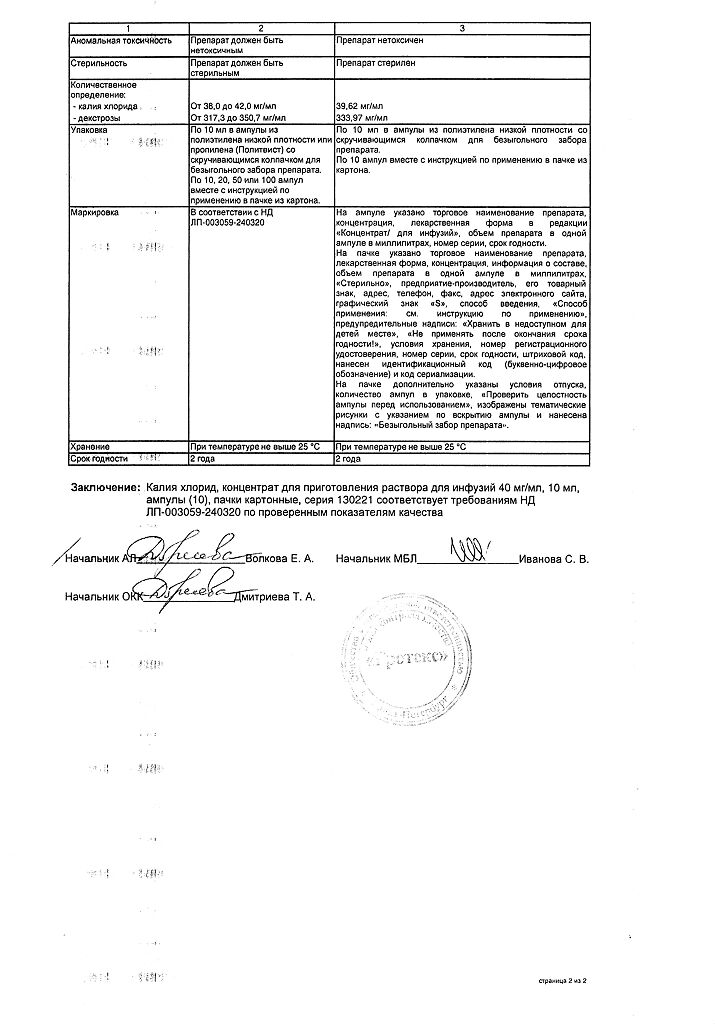
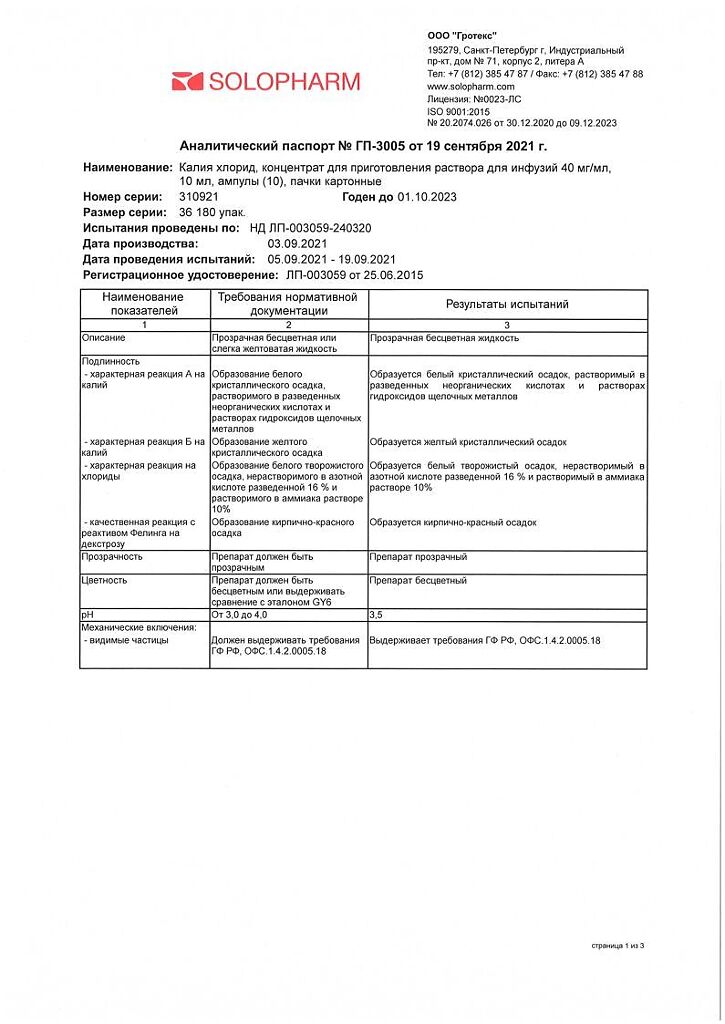
Composition
Composition
potassium chloride – 40 mg
dextrose (or glucose in terms of dry matter – 334 mg
hydrochloric acid solution 0.1 M – to pH 3.0-4.0
water for injection – up to 1 ml
Contraindications
Contraindications
– Hypersensitivity to the active ingredient and auxiliary components of the drug, to acetylsalicylic acid or other NSAIDs.
– Complete or incomplete combination of bronchial asthma, recurrent polyposis of the nose and paranasal sinuses, angioedema or urticaria caused by intolerance to acetylsalicylic acid or other NSAIDs due to the existing likelihood of cross-sensitivity (including history).
– Erosive and ulcerative lesions of the stomach and duodenum in the acute stage or recently suffered.
– Inflammatory bowel diseases – Crohn’s disease or ulcerative colitis in the acute stage.
– Severe liver and heart failure.
– Severe renal failure (if hemodialysis is not performed, creatinine clearance less than 30 ml/min), progressive kidney disease, including confirmed hyperkalemia.
– Active liver disease.
– Active gastrointestinal bleeding, recent cerebrovascular bleeding or an established diagnosis of diseases of the blood coagulation system.
– Age up to 18 years.
– Therapy of perioperative pain during coronary artery bypass surgery.
– Concomitant therapy with anticoagulants, as there is a risk of formation of intramuscular hematomas.
– Pregnancy.
– Breastfeeding period.
With caution:
– History of gastrointestinal (GIT) diseases (presence of Helicobacter pylori infection).
– Congestive heart failure.
– Renal failure (creatinine clearance 30-60 ml/min).
– Coronary heart disease.
– Cerebrovascular diseases.
– Dyslipidemia/hyperlipidemia.
– Diabetes mellitus.
– Concomitant therapy with the following drugs: anticoagulants, oral glucocorticosteroids, antiplatelet agents, selective serotonin reuptake inhibitors.
– Peripheral arterial diseases.
– Old age.
– Long-term use of NSAIDs.
– Smoking.
– Frequent drinking of alcohol.
Side Effects
Side Effects
From the nervous system, paresthesia, muscle weakness, confusion.
Interaction
Interaction
Pharmaceutically compatible with solutions of cardiac glycosides (improves their tolerability).
Overdose
Overdose
Symptoms: hyperkalemia (muscle hypotonicity, paresthesia of the extremities, slowing of atrioventricular conduction, arrhythmias, cardiac arrest).
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature from 0 to +30 ° C.
Shelf life
Shelf life
3 years. Do not use after expiration date.
Manufacturer
Manufacturer
Grotex LLC, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature of 0 to +30 ° C. |
| Manufacturer | Grotex Ltd, Russia |
| Medication form | concentrate for preparation of infusion solution |
| Brand | Grotex Ltd |
Related products
Buy Potassium chloride, 40 mg/ml concentrate 10 ml 10 pcs with delivery to USA, UK, Europe and over 120 other countries.