No products in the cart.
Description
Blocker of histamine H1-receptors. It has anti-allergic and antipruritic effects. Reduces increased capillary permeability associated with allergic reactions. When applied to the skin it reduces itching and irritation caused by skin allergic reactions.
The drug also has a pronounced local anesthetic effect.
Indications
Indications
Skin itching of various origins (except those associated with cholestasis), for example: itchy dermatoses, eczema, urticaria, insect bites. Sunburn, household and industrial burns (mild).
Pharmacological effect
Pharmacological effect
Antihistamine, antiallergic and antipruritic agent. H1-histamine receptor blocker, is a competitive histamine antagonist. The drug reduces increased capillary permeability associated with allergic reactions. When applied to the skin, Fenistil® gel reduces itching and irritation caused by allergic skin reactions. The drug also has a pronounced local anesthetic effect. It also has antikinin and weak anticholinergic effects. When used externally, thanks to the gel base, it has a quick onset of action (within a few minutes) and a slight cooling effect. Maximum effect – after 1 – 4 hours.
Special instructions
Special instructions
In children from 1 month to 2 years, the drug is used after consultation with a doctor.
In infants and young children, the drug should not be used on large areas of the skin, especially if there is inflammation or bleeding.
In case of severe itching or if large areas of skin are affected, the drug can be used only after consultation with a doctor.
When using Fenistil® gel on large areas of the skin, exposure to sunlight should be avoided.
If during the period of use of Fenistil® gel the severity of the symptoms of the disease does not decrease or, on the contrary, intensifies, you should consult a doctor.
Ineffective for itching associated with cholestasis.
The drug contains propylene glycol and benzalkonium chloride, which can cause local allergic reactions.
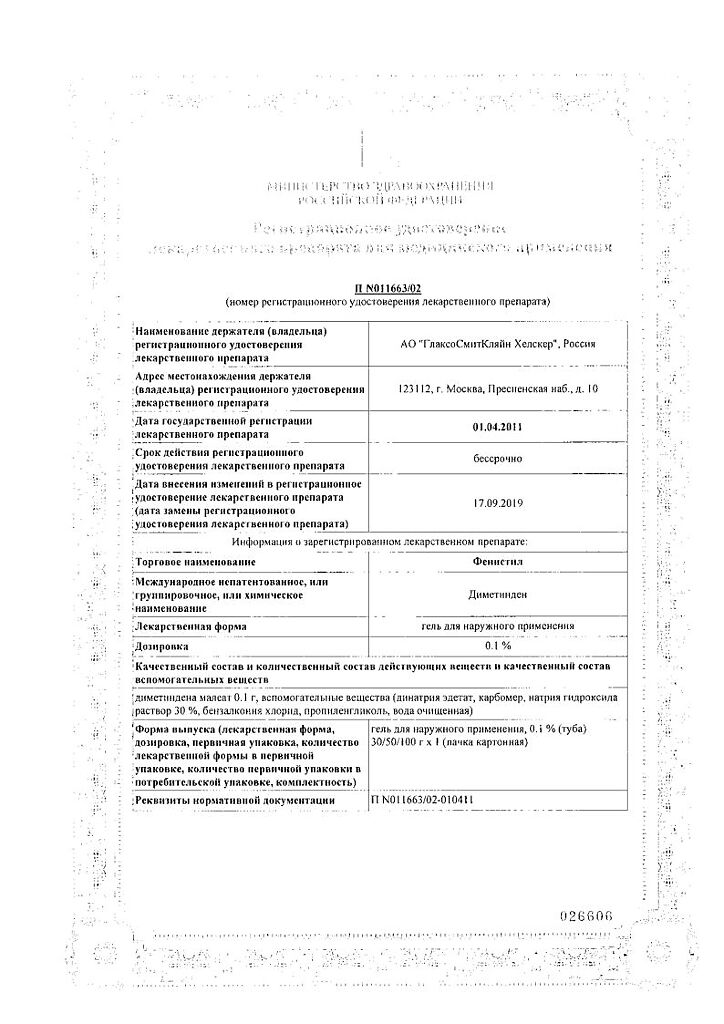
Active ingredient
Active ingredient
Dimetinden
Composition
Composition
Active substance: dimethindene maleate. 100 g of gel for external use contain 0.1 g of dimethindene maleate.
Excipients: disodium edetate 0.05 g, carbomer about 0.9 g, sodium hydroxide solution 30% about 1.0 g, benzalkonium chloride 0.005 g, propylene glycol 15.0 g, purified water about 82.9 g.
Pregnancy
Pregnancy
The use of the drug in the first three months of pregnancy is possible only after consultation with a doctor.
In the second and third trimesters of pregnancy, as well as during lactation, Fenistil® gel should not be used on large areas of the skin, especially in the presence of inflammation or bleeding.
Nursing mothers should not apply the drug to the nipples of the mammary glands.
Contraindications
Contraindications
Hypersensitivity to dimethindene and other components included in the drug, angle-closure glaucoma, prostatic hyperplasia, children under 1 month of age, especially premature infants.
Side Effects
Side Effects
Classification of the frequency of occurrence of adverse reactions: very often (≥1/10); often (≥1/100, <1/10); uncommon (≥ 1/1000, < 1/100); rare (≥1/10000, <1/1000); very rare (< 1/10000), including isolated reports and reactions with unknown frequency (cannot be calculated from available data).
Skin and subcutaneous tissue disorders: Uncommon: dry, burning skin. Very rare (post-registration data): allergic dermatitis, including skin rash, itching.
If any of the side effects indicated in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Interaction
Interaction
There are no known drug interactions for Fenistil® gel.
Overdose
Overdose
If a large amount of the drug is accidentally ingested, symptoms characteristic of an overdose of H1-histamine receptor blocker drugs may occur, including depression of the functions of the central nervous system, drowsiness (mainly in adults), stimulation of the functions of the central nervous system, antimuscarinic effects (especially in children), including increased excitability, ataxia, hallucinations, tonic-clonic convulsions, mydriasis, dry mouth, “flushes” of blood to the face, urinary retention and fever. This may be followed by a drop in blood pressure.
Treatment: No specific antidote is known. The usual emergency measures should be taken: when ingested – taking activated charcoal, saline laxatives; if necessary, take measures to maintain the function of the cardiovascular and respiratory systems.
Vasoconstrictors can be used to treat arterial hypotension. Do not exceed the recommended dose of Fenistil® gel.
In case of accidental overdose, tell your doctor immediately.
Clinical pharmacology
Clinical pharmacology
When used externally, it penetrates well into the skin, systemic bioavailability is 10%.
Short product description
Short product description
Fenistil Gel reduces itching and irritation caused by allergic skin reactions.
Recommendations for use
Recommendations for use
Externally. The gel is applied to the affected area of the skin 2 – 4 times a day. In cases of severe itching or widespread skin lesions, simultaneous use of oral forms is recommended.
Storage conditions
Storage conditions
At a temperature not exceeding 25°C. Keep out of the reach of children.
Shelf life
Shelf life
2 years. Do not use after expiration date.
Manufacturer
Manufacturer
GSK Consumer Healthcare S.A., Switzerland
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children, the gel at a temperature not exceeding 25°C. |
| Manufacturer | GSC Consumer Healthcare S.A., Switzerland |
| Medication form | gel for external use |
| Brand | GSC Consumer Healthcare S.A. |
Other forms…
Related products
Buy Phenystil, gel 0.1% 50 g with delivery to USA, UK, Europe and over 120 other countries.