No products in the cart.
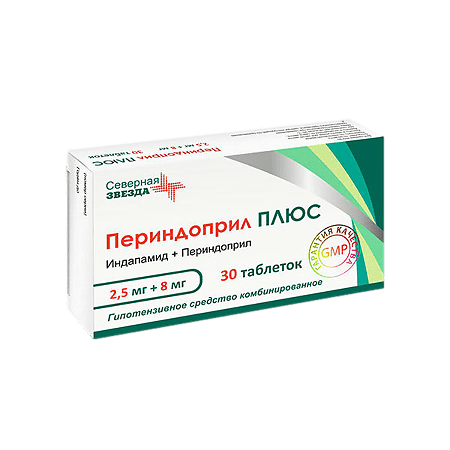
Perindopril PLUS, tablets 2.5mg+8 mg 30 pcs
€12.25 €10.21
Description
Perindopril PLUS is a combined drug containing indapamide and perindopril erbumin. Pharmacological properties of the drug Perindopril PLUS combine individual properties of each of its active components.
Mechanism of Action
Perindopril PLUS
The combination of indapamide and perindopril enhances the antihypertensive effects of each.
Perindopril
Perindopril is an inhibitor of the enzyme that converts angiotensin I to angiotensin II (angiotensin-converting enzyme (ACE) inhibitor). ACE, or kininase II, is an exopeptidase that performs both the conversion of angiotensin I vasoconstrictor angiotensin II and the degradation of bradykinin, which has a vasodilatory effect, to an inactive heptapeptide.
The result is perindopril:
- reduces aldosterone secretion;
- by the principle of negative feedback, it increases plasma renin activity;
Perindopril normalizes myocardial function by reducing preload and postload.
In a study of hemodynamic parameters in patients with chronic heart failure (CHF), it was found:
- reduced filling pressures in the left and right ventricles of the heart;
- reduced PPS;
- increased cardiac output and increased cardiac index;
- increased muscle peripheral blood flow.
Indapamide
Indapamide belongs to the group of sulfonamides and has pharmacological properties similar to thiazide diuretics.
Indapamide inhibits reabsorption of sodium ions in the cortical segment of the Genle loop, which leads to increased renal excretion of sodium ions, chloride ions and to a lesser extent sodium and magnesium ions, thereby increasing diuresis and reducing blood pressure (BP).
Antihypertensive effects
Perindopril PLUS
The drug Perindopril PLUS has dose-dependent antihypertensive effects on both diastolic and systolic BP in both standing and lying positions.
The antihypertensive effect lasts for 24 h. Stable therapeutic effect develops in less than 1 month from the beginning of therapy and is not accompanied by tachyphylaxis. Discontinuation of therapy does not cause “withdrawal” syndrome.
The drug Perindopril PLUS reduces the degree of left ventricular hypertrophy (LVH), improves arterial elasticity, decreases PEEP, has no effect on lipid metabolism (total cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, triglycerides).
The combination of perindopril and indapamide has been shown to have an effect on HLV compared with enalapril. In patients with arterial hypertension and HLV treated with perindopril erbumin 2 mg/indapamide 0.625 mg or enalapril at a dose of 10 mg once daily and when the perindopril erbumin dose was increased to 8 mg and indapamide to 2,5 mg, or enalapril to 40 mg once daily, there was a more significant reduction in left ventricular mass index (LVMI) of the perindopril/indapamide group compared with the enalapril group. The most significant effect on BMI was noted with perindopril erbumin 8 mg/indapamide 2.5 mg.
There was also a more pronounced antihypertensive effect on combined therapy with perindopril and indapamide compared with enalapril.
Perindopril
Perindopril is effective in therapy of arterial hypertension of any severity. Its antihypertensive effect reaches its maximum 4-6 hours after a single oral dose and lasts for 24 hours. Twenty-four hours after the drug administration there is a significant (about 80%) residual inhibition of ACE.
Perindopril has antihypertensive effect in patients with both low and normal plasma renin activity. Concomitant administration of thiazide diuretics increases the severity of antihypertensive effect. In addition, combination of ACE inhibitor and thiazide diuretic also leads to decrease of risk of hypokalemia during diuretic therapy.
Double blockade of the renin-angiotensin-aldosterone system (RAAS)
There are data from clinical studies of combined therapy with ACE inhibitor with angiotensin II receptor antagonists (ARA II).
There have been clinical studies involving patients with a history of cardiovascular or cerebrovascular disease, or type 2 diabetes with confirmed target organ damage, and studies involving patients with type 2 diabetes and diabetic nephropathy.
These studies found no significant positive effect on the occurrence of renal and/or cardiovascular events and mortality rates in patients receiving combination therapy, while the risk of hyperkalemia, acute renal failure and/or arterial hypotension increased compared to patients receiving monotherapy.
With consideration of the similar within-group pharmacodynamic properties of ACE inhibitors and ARA II, these results would be expected for any other drug interaction between ACE inhibitors and ARA II classes.
The use of ACE inhibitors in combination with angiotensin II receptor antagonists is therefore contraindicated in patients with diabetic nephropathy.
There are data from a clinical trial examining the beneficial effects of adding aliskiren to standard therapy with an ACE inhibitor or ARA II in patients with type 2 diabetes mellitus and chronic kidney disease or cardiovascular disease, or who have a combination of these conditions.
The study was stopped early due to an increased risk of adverse outcomes. Cardiovascular death and stroke occurred more frequently in the group of patients receiving aliskiren compared to the placebo group. Also adverse events and serious adverse events of special interest (hyperkalemia, arterial hypotension and renal dysfunction) were reported more frequently in the aliskiren group than in the placebo group.
Indapamide
The antihypertensive effect is seen when the drug is used in doses that have minimal diuretic effect. The antihypertensive effect of indapamide is associated with improvement of elastic properties of large arteries, reduction of PPS.
Indapamide decreases VLDL, does not influence plasma lipid concentrations: triglycerides, total cholesterol, LDL, HDL; carbohydrate metabolism (including in patients with concomitant diabetes).
Indications
Indications
Essential hypertension.
Pharmacological effect
Pharmacological effect
The drug Perindopril PLUS is a combination drug containing indapamide and perindopril erbumine. The pharmacological properties of the drug Perindopril PLUS combine the individual properties of each of its active components.
Mechanism of action
Perindopril PLUS
The combination of indapamide and perindopril enhances the antihypertensive effect of each of them.
Perindopril
Perindopril is an inhibitor of the enzyme that converts angiotensin I to angiotensin II (angiotensin-converting enzyme (ACE) inhibitor). ACE, or kininase II, is an exopeptidase that carries out both the conversion of angiotensin I to the vasoconstrictor angiotensin II and the destruction of bradykinin, which has a vasodilator effect, to an inactive heptapeptide.
As a result, perindopril:
reduces the secretion of aldosterone;
according to the principle of negative feedback, it increases the activity of renin in the blood plasma;
with long-term use, it reduces total peripheral vascular resistance (TPVR), which is mainly due to the effect on the vessels in the muscles and kidneys. These effects are not accompanied by sodium and fluid retention or the development of reflex tachycardia.
Perindopril normalizes myocardial function, reducing preload and afterload.
When studying hemodynamic parameters in patients with chronic heart failure (CHF), it was revealed:
decreased filling pressure in the left and right ventricles of the heart;
decrease in OPSS;
increased cardiac output and increased cardiac index;
increased muscle peripheral blood flow.
Indapamide
Indapamide belongs to the group of sulfonamides; its pharmacological properties are similar to thiazide diuretics.
Indapamide inhibits the reabsorption of sodium ions in the cortical segment of the loop of Henle, which leads to an increase in the excretion of sodium, chlorine and, to a lesser extent, sodium and magnesium ions by the kidneys, thereby increasing diuresis and reducing blood pressure (BP).
Antihypertensive effect
Perindopril PLUS
The drug Perindopril PLUS has a dose-dependent antihypertensive effect on both diastolic and systolic blood pressure in both the standing and lying positions.
The antihypertensive effect persists for 24 hours. A stable therapeutic effect develops in less than 1 month from the start of therapy and is not accompanied by tachyphylaxis. Stopping treatment does not cause withdrawal syndrome.
The drug Perindopril PLUS reduces the degree of left ventricular hypertrophy (LVH), improves arterial elasticity, reduces peripheral vascular resistance, and does not affect lipid metabolism (total cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, triglycerides).
The effect of the use of a combination of perindopril and indapamide on LVH in comparison with enalapril has been proven. In patients with arterial hypertension and LVH who received therapy with perindopril erbumine 2 mg/indapamide 0.625 mg or enalapril at a dose of 10 mg once daily, and with an increase in the dose of perindopril erbumine to 8 mg and indapamide to 2.5 mg, or enalapril to 40 mg once daily, a more significant decrease in mass index was noted left ventricular (LVMI) group in the perindopril/indapamide group compared with the enalapril group. In this case, the most significant effect on LVMI was observed with the use of perindopril erbumine 8 mg/indapamide 2.5 mg.
A more pronounced antihypertensive effect was also noted during combination therapy with perindopril and indapamide compared to enalapril.
Perindopril
Perindopril is effective in the treatment of arterial hypertension of any severity. The antihypertensive effect of the drug reaches its maximum 4-6 hours after a single oral dose and persists for 24 hours. 24 hours after taking the drug, pronounced (about 80%) residual ACE inhibition is observed.
Perindopril has an antihypertensive effect in patients with both low and normal plasma renin activity. The simultaneous administration of thiazide diuretics increases the severity of the antihypertensive effect. In addition, the combination of an ACE inhibitor and a thiazide diuretic also reduces the risk of hypokalemia while taking diuretics.
Dual blockade of the renin-angiotensin-aldosterone system (RAAS)
There are data from clinical studies of combination therapy using an ACE inhibitor with angiotensin II receptor antagonists (ARA II).
Clinical studies were conducted in patients with a history of cardiovascular or cerebrovascular disease, or type 2 diabetes mellitus accompanied by confirmed target organ damage, as well as studies in patients with type 2 diabetes mellitus and diabetic nephropathy.
These studies did not reveal a significant positive effect on renal and/or cardiovascular events and mortality rates in patients receiving combination therapy, while the risk of hyperkalemia, acute renal failure and/or hypotension increased compared with patients receiving monotherapy.
Taking into account the similar intragroup pharmacodynamic properties of ACE inhibitors and ARB II, these results can be expected for the interaction of any other drugs, representatives of the classes of ACE inhibitors and ARA II.
Therefore, the use of ACE inhibitors in combination with angiotensin II receptor antagonists in patients with diabetic nephropathy is contraindicated.
There is clinical trial data examining the beneficial effects of adding aliskiren to standard therapy with an ACE inhibitor or angiotensin-converting enzyme II inhibitor in patients with type 2 diabetes mellitus and chronic kidney disease or cardiovascular disease, or a combination of these diseases.
The study was stopped early due to an increased risk of adverse outcomes. Cardiovascular death and stroke occurred more frequently in the aliskiren group compared to the placebo group. Also, adverse events and serious adverse events of special interest (hyperkalemia, hypotension, and renal dysfunction) were reported more frequently in the aliskiren group than in the placebo group.
Indapamide
The antihypertensive effect occurs when the drug is used in doses that have a minimal diuretic effect. The antihypertensive effect of indapamide is associated with an improvement in the elastic properties of large arteries and a decrease in peripheral vascular resistance.
Indapamide reduces LVH and does not affect the concentration of lipids in the blood plasma: triglycerides, total cholesterol, LDL, HDL; carbohydrate metabolism (including in patients with concomitant diabetes mellitus).
Special instructions
Special instructions
Renal dysfunction
Therapy is contraindicated in patients with moderate and severe renal failure (creatinine clearance less than 60 ml/min). In some patients with arterial hypertension without previous obvious renal impairment, laboratory signs of functional renal failure may appear during therapy. In this case, treatment should be stopped. In the future, you can resume combination therapy using low doses of a combination of indapamide and perindopril, or use only one of the drugs.
Such patients require regular monitoring of potassium levels and creatinine concentrations in the blood serum – 2 weeks after the start of therapy and every 2 months thereafter. Renal failure occurs more often in patients with severe chronic heart failure or underlying renal impairment, including renal artery stenosis. The drug Perindopril PLUS is not recommended in cases of bilateral renal artery stenosis or stenosis of the artery of a single functioning kidney.
Arterial hypotension and water-electrolyte imbalance
In the case of initial hyponatremia, there is a risk of sudden development of arterial hypotension, especially in patients with renal artery stenosis. Therefore, during dynamic monitoring of patients, attention should be paid to possible symptoms of dehydration and decreased electrolyte levels in the blood plasma, for example, after diarrhea or vomiting. Such patients require regular monitoring of blood plasma electrolyte levels.
In case of severe arterial hypotension, intravenous administration of 0.9% sodium chloride solution may be required.
Transient arterial hypotension is not a contraindication for continued therapy. After restoration of circulating blood volume and blood pressure, therapy can be resumed using low doses of drugs, or only one of the drugs can be used.
Potassium content
The combined use of perindopril and indapamide does not prevent the development of hypokalemia, especially in patients with diabetes mellitus or renal failure. As with any antihypertensive drug or diuretic, regular monitoring of potassium levels in the blood plasma is necessary.
Excipients
It should be taken into account that the excipients of the drug include lactose monohydrate. The drug Perindopril PLUS should not be prescribed to patients with hereditary galactose intolerance, lactase deficiency and glucose-galactose malabsorption.
Lithium preparations
The simultaneous use of the drug Perindopril PLUS with lithium preparations is not recommended.
Childhood
The drug should not be prescribed to children and adolescents under the age of 18 years due to the lack of data on the effectiveness and safety of the use of indapamide and perindopril, both separately and together, in patients in this age group.
Perindopril
Dual blockade of the renin-angiotensin-aldosterone system (RAAS)
There is evidence of an increased risk of arterial hypotension, hyperkalemia and renal dysfunction (including acute renal failure) when ACE inhibitors are used simultaneously with ARB II or aliskiren. Therefore, double blockade of the RAAS by combining an ACE inhibitor with an ARB II or aliskiren is not recommended.
If a double blockade is absolutely necessary, then this should be performed under the strict supervision of a specialist with regular monitoring of renal function, plasma electrolytes and blood pressure.
The use of ACE inhibitors in combination with ARA II receptor antagonists is contraindicated in patients with diabetic nephropathy and is not recommended in other patients.
Potassium-sparing diuretics, potassium supplements, potassium-containing table salt substitutes and food supplements
The simultaneous administration of perindopril and potassium-sparing diuretics, as well as potassium supplements, potassium-containing table salt substitutes and food additives is not recommended.
Neutropenia/agranulocytosis/thrombocytopenia
There are reports of the development of neutropenia/agranulocytosis, thrombocytopenia and anemia while taking ACE inhibitors. In patients with normal renal function and without concomitant risk factors, neutropenia rarely occurs. Perindopril should be used with extreme caution against the background of systemic connective tissue diseases (including systemic lupus erythematosus, scleroderma), as well as while taking immunosuppressants, allopurinol or procainamide, or a combination of these factors, especially in patients with initially impaired renal function.
Some patients developed severe infectious diseases, in some cases resistant to intensive antibiotic therapy. When prescribing perindopril to such patients, it is recommended to periodically monitor the number of leukocytes in the blood. Patients should report any signs of infectious diseases (eg, sore throat, fever) to their doctor.
Anemia
Anemia may develop in patients after kidney transplantation or in those on hemodialysis. In this case, the decrease in hemoglobin is greater, the higher its initial value. This effect does not appear to be dose-dependent, but may be related to the mechanism of action of ACE inhibitors.
A slight decrease in hemoglobin occurs during the first 6 months, then it remains stable and is completely restored after discontinuation of the drug. In such patients, treatment can be continued, but hematological tests should be performed regularly.
Hypersensitivity/angioedema
When taking ACE inhibitors, including perindopril, in rare cases, the development of angioedema of the face, extremities, lips, tongue, vocal folds and/or larynx may occur. This can happen at any time during therapy. If symptoms appear, Perindopril PLUS should be stopped immediately and the patient should be observed until signs of swelling have completely disappeared. If the swelling affects only the face and lips, it usually goes away on its own, although antihistamines can be used as symptomatic therapy.
Angioedema, accompanied by swelling of the larynx, can be fatal. Swelling of the tongue, vocal folds, or larynx can lead to airway obstruction. If such symptoms appear, you should immediately begin appropriate therapy, for example, subcutaneously administer epinephrine (adrenaline) at a dilution of 1:1000 (0.3 – 0.5 ml) and/or ensure airway patency.
A higher risk of developing angioedema has been reported in black patients.
Patients with a history of angioedema not associated with taking ACE inhibitors may have an increased risk of developing it when taking drugs of this group.
In rare cases, angioedema of the intestine develops during therapy with ACE inhibitors. In this case, patients experienced abdominal pain as an isolated symptom or in combination with nausea and vomiting, in some cases without previous angioedema of the face and with normal levels of C-1 esterase. Diagnosis was made using abdominal computed tomography, ultrasound, or at the time of surgery. Symptoms resolved after discontinuation of ACE inhibitors. Therefore, in patients with abdominal pain receiving ACE inhibitors, when carrying out differential diagnosis, it is necessary to take into account the possibility of developing angioedema of the intestine.
mTOR (mammalian target of rapamycin) inhibitors (eg, sirolimus, everolimus, temsirolimus)
Patients receiving concomitant therapy with mTOR inhibitors may have an increased risk of developing angioedema (including swelling of the airways or tongue with or without respiratory impairment).
Anaphylactoid reactions during desensitization
There are isolated reports of the development of prolonged, life-threatening anaphylactoid reactions in patients receiving ACE inhibitors during desensitizing therapy with hymenopteran insect venom (bees, wasps). ACE inhibitors should be used with caution in patients prone to allergic reactions undergoing desensitization procedures. Prescription of an ACE inhibitor should be avoided in patients receiving immunotherapy with hymenoptera venom. However, an anaphylactoid reaction can be avoided by temporarily discontinuing the ACE inhibitor at least 24 hours before the start of the desensitization procedure.
Anaphylactoid reactions during LDL apheresis
In rare cases, life-threatening anaphylactoid reactions have developed in patients receiving ACE inhibitors during LDL apheresis using dextran sulfate. To prevent an anaphylactoid reaction, ACE inhibitor therapy should be temporarily discontinued before each apheresis procedure.
Hemodialysis
Anaphylactoid reactions have been reported in patients receiving ACE inhibitors during hemodialysis using high-flux membranes (eg, AN69®). Therefore, it is advisable to use a different type of membrane or use an antihypertensive agent of a different pharmacotherapeutic group.
Cough
During therapy with an ACE inhibitor, a dry persistent cough may occur, which disappears after discontinuation of drugs of this group and disappears after their discontinuation. If a patient develops a dry cough, one should be aware of the possible iatrogenic nature of this symptom. If the attending physician believes that ACE inhibitor therapy is necessary for the patient, it is possible to continue taking the drug.
Risk of arterial hypotension and/or renal failure (in patients with heart failure, fluid and electrolyte imbalance, etc.)
In some pathological conditions, significant activation of the RAAS may be observed, especially with severe hypovolemia and a decrease in the content of electrolytes in the blood plasma (due to a salt-free diet or long-term use of diuretics), in patients with initially low blood pressure, renal artery stenosis, chronic heart failure or cirrhosis of the liver with edema and ascites.
The use of ACE inhibitors causes blockade of the RAAS and therefore may be accompanied by a sharp decrease in blood pressure and/or an increase in the concentration of creatinine in the blood plasma, indicating the development of functional renal failure. These phenomena are more often observed when taking the first dose of the drug and during the first two weeks of therapy. In rare cases, these conditions develop acutely and during other periods of therapy. In such cases, it is recommended to restart therapy at a lower dose and then gradually increase the dose.
Old age
Before starting to take perindopril, it is necessary to assess the functional activity of the kidneys and the content of potassium ions in the blood plasma. At the beginning of therapy, the dose of the drug is selected taking into account the degree of reduction in blood pressure, especially in the case of dehydration and loss of electrolytes. Such measures help to avoid a sharp decrease in blood pressure.
Atherosclerosis
The risk of arterial hypotension exists in all patients, however, special care should be taken when using the drug in patients with coronary heart disease and cerebrovascular insufficiency. In such patients, treatment should begin with low doses of the drug.
Renovascular hypertension
The treatment method for renovascular hypertension is revascularization. However, the use of ACE inhibitors may have a positive effect in this category of patients, both awaiting surgery and in cases where surgery is not possible.
Treatment with Perindopril PLUS is not indicated in patients with diagnosed or suspected renal artery stenosis, because Therapy should be started in a hospital setting with lower doses of the combination of indapamide and perindopril.
Heart failure/severe heart failure
In patients with chronic heart failure (NYHA functional class IV), treatment should begin with lower doses of the combination of indapamide and perindopril and under close medical supervision.
Patients with arterial hypertension and coronary heart disease should not stop taking beta-blockers: an ACE inhibitor should be added to beta-blocker therapy.
Diabetes mellitus
In patients with type 1 diabetes mellitus, a spontaneous increase in potassium levels in the blood is possible. Treatment of such patients with the drug Perindopril PLUS is not indicated, since it should begin with minimal doses and be under constant medical supervision.
During the first month of therapy with ACE inhibitors, plasma glucose concentrations should be carefully monitored in patients with diabetes mellitus receiving oral hypoglycemic agents or insulin.
Ethnic differences
Perindopril, like other ACE inhibitors. has a clearly less pronounced antihypertensive effect in patients of the Negroid race compared to representatives of other races. This difference may be due to the fact that black patients with arterial hypertension are more likely to have low renin activity.
Surgery / General anesthesia
Carrying out general anesthesia against the background of ACE inhibitors can lead to a pronounced decrease in blood pressure, especially when using general anesthesia agents that have an antihypertensive effect.
It is recommended, if possible, to stop taking long-acting ACE inhibitors, including perindopril, the day before surgery. It is necessary to warn the anesthesiologist that the patient is taking ACE inhibitors.
Aortic or mitral stenosis / Hypertrophic obstructive cardiomyopathy
ACE inhibitors should be prescribed with caution to patients with left ventricular outflow tract obstruction.
Liver failure
In rare cases, cholestatic jaundice occurs while taking ACE inhibitors. As this syndrome progresses, fulminant liver necrosis develops, sometimes with death. The mechanism of development of this syndrome is unclear. If jaundice appears or a significant increase in liver enzyme activity occurs while taking ACE inhibitors, the patient should stop taking the ACE inhibitor and consult a doctor.
Hyperkalemia
Hyperkalemia may develop during treatment with ACE inhibitors, including perindopril. Risk factors for hyperkalemia are renal failure, impaired renal function, age over 70 years, diabetes mellitus, certain concomitant conditions (dehydration, acute cardiac decompensation, metabolic acidosis), concomitant use of potassium-sparing diuretics (such as spironolactone and its derivative eplerenone, triamterene, amiloride), as well as potassium supplements or potassium-containing substitutes for table salt, as well as the use of other drugs that help increase potassium levels in the blood plasma (for example, heparins, ACE inhibitors, angiotensin II receptor antagonists, acetylsalicylic acid at a dose of 3 g / day or more, cyclooxygenase-2 (COX-2) inhibitors and non-selective NSAIDs, immunosuppressants such as cyclosporine or tacrolimus, trimethoprim).
The use of potassium supplements, potassium-sparing diuretics, and potassium-containing table salt substitutes can lead to a significant increase in potassium levels in the blood, especially in patients with reduced renal function.
Hyperkalemia can cause serious, sometimes fatal, abnormal heart rhythms. If simultaneous use of the above drugs is necessary, treatment should be carried out with caution against the background of regular monitoring of potassium levels in the blood serum.
Indapamide
Hepatic encephalopathy
In the presence of liver dysfunction, taking thiazide and thiazide-like diuretics can lead to the development of hepatic encephalopathy. In such a situation, you should immediately stop taking the diuretic.
Water and electrolyte balance
Content of sodium ions in blood plasma
The content of sodium ions in the blood plasma must be determined before starting treatment, and then regularly monitored while taking the drug.
Hyponatremia at the initial stage may not be accompanied by clinical symptoms, so regular laboratory monitoring is necessary. More frequent monitoring of sodium ion levels is indicated for patients with liver cirrhosis and elderly patients. Treatment with any diuretics can cause hyponatremia, sometimes with very serious consequences. Hyponatremia accompanied by hypovolemia can lead to dehydration and orthostatic hypotension.
A simultaneous decrease in the content of chloride ions can lead to the development of secondary compensatory metabolic alkalosis: the frequency of its occurrence and the severity of the manifestation are insignificant.
Content of potassium ions in blood plasma
Therapy with thiazide and thiazide-like diuretics is associated with a risk of hypokalemia. Hypokalemia (less than 3.4 mmol/l) should be avoided in the following categories of high-risk patients: elderly patients, malnourished patients (both those receiving and not receiving concomitant drug therapy), patients with cirrhosis of the liver (with edema and ascites), coronary artery disease, heart failure. Hypokalemia in these patients increases the toxic effect of cardiac glycosides and increases the risk of developing arrhythmia.
Patients with a prolonged QT interval, either congenital or drug-induced, are also at increased risk.
Hypokalemia, like bradycardia, contributes to the development of severe cardiac arrhythmias, especially pirouette-type arrhythmias, which can be fatal. In all the cases described above, more frequent monitoring of the content of potassium ions in the blood plasma is necessary. The first measurement of potassium ion content should be carried out within the first week from the start of therapy.
If hypokalemia is detected, appropriate correction should be made.
Content of calcium ions in blood plasma
Thiazide and thiazide-like diuretics may reduce the excretion of calcium ions by the kidneys, leading to a slight and temporary increase in plasma calcium. Severe hypercalcemia may be a consequence of previously undiagnosed hyperparathyroidism. Before studying the function of the parathyroid glands, you should stop taking diuretics.
Plasma glucose concentration
It is necessary to monitor blood glucose concentrations in patients with diabetes mellitus, especially in the presence of hypokalemia.
Uric acid
When the concentration of uric acid in the blood plasma increases during therapy, the frequency of gout attacks may increase.
Diuretics and kidney function
Thiazide and thiazide-like diuretics are fully effective only in patients with normal or slightly impaired renal function (plasma creatinine concentration in adults below 25 mg/l or 220 µmol/l).
In elderly patients, plasma creatinine levels should be assessed taking into account age, weight and sex, according to the Cockroft formula:
Creatinine clearance (CC) = (140 – age) x weight / 0.814 x plasma creatinine concentration
where: age in years, weight in kg, plasma creatinine concentration in µmol/l.
The formula is suitable for older men; for older women, the result should be multiplied by a factor of 0.85.
At the beginning of diuretic treatment in patients, due to hypovolemia (due to the excretion of water and sodium ions), a temporary decrease in glomerular filtration rate and an increase in the concentration of urea and creatinine in the blood plasma may be observed. This transient functional renal failure is not dangerous for patients with initially normal renal function, but its severity may increase in patients with renal failure.
Photosensitivity
Cases of photosensitivity reactions have been reported while taking thiazide and thiazide-like diuretics. If photosensitivity reactions develop while taking the drug, treatment should be discontinued. If it is necessary to continue diuretic therapy, it is recommended to protect the skin from exposure to sunlight or artificial ultraviolet rays.
Athletes
Indapamide may give a positive reaction during doping control.
Acute myopia and secondary angle-closure glaucoma
Sulfonamides and their derivatives can cause the development of idiosyncratic reactions leading to temporary (transient) myopia and acute angle-closure glaucoma. Without proper treatment, acute angle-closure glaucoma can lead to vision loss. First of all, you need to stop taking the drug as soon as possible. If intraocular pressure continues to be high, immediate medical or surgical treatment may be required. Risk factors that may lead to the development of acute angle-closure glaucoma include a history of allergies to sulfonamides or penicillin.
Impact on the ability to drive vehicles. Wed and fur.:
Care must be taken when driving vehicles and other technical devices that require increased attention and speed of psychomotor reactions.
Active ingredient
Active ingredient
Indapamide, Perindopril
Composition
Composition
1 tablet contains:
active ingredients:
indapamide – 2.5 mg,
perindopril erbumine – 8.0 mg;
excipients: microcrystalline cellulose 102 – 141.5 mg; croscarmellose sodium (primellose) – 6.0 mg; pregelatinized corn starch (starch 1500) – 30.0 mg; sodium bicarbonate – 8.0 mg; magnesium stearate – 2.0 mg; colloidal silicon dioxide anhydrous (aerosil anhydrous) – 2.0 mg.
Contraindications
Contraindications
Perindopril
Hypersensitivity to perindopril and other ACE inhibitors;
History of angioedema (Quincke’s edema) while taking other ACE inhibitors;
Hereditary/idiopathic angioedema;
Pregnancy and breastfeeding period;
Concomitant use with aliskiren and medicinal products containing aliskiren in patients with diabetes mellitus and/or moderate or severe renal impairment (glomerular filtration rate (GFR) less than 60 ml/min/1.73 m2 body surface area.
Concomitant use with angiotensin II receptor antagonists (ARA II) in patients with diabetic nephropathy.
Age up to 18 years (efficacy and safety have not been established).
Indapamide
Hypersensitivity to indapamide and other sulfonamides;
Moderate to severe renal failure (creatinine clearance (CC) less than 60 ml/min);
Severe liver failure;
Hepatic encephalopathy;
Hypokalemia;
Concomitant use with non-antiarrhythmic drugs that can cause polymorphic ventricular tachycardia of the “pirouette” type;
Breastfeeding period;
Age up to 18 years (efficacy and safety have not been established).
Perindopril PLUS
Hypersensitivity to the excipients included in the drug;
Due to the lack of sufficient clinical experience, the drug Perindopril PLUS should not be used in patients on hemodialysis, as well as in patients with untreated decompensated heart failure;
Age up to 18 years (efficacy and safety have not been established);
The presence of lactase deficiency, galactosemia or glucose-galactose malabsorption syndrome, lactose intolerance (the drug contains lactose).
With caution:
Systemic connective tissue diseases (including systemic lupus erythematosus, scleroderma); therapy with immunosuppressants (risk of developing neutropenia, agranulocytosis); combined use with lithium preparations, gold preparations, non-steroidal anti-inflammatory drugs (NSAIDs), baclofen, corticosteroids, drugs that can cause prolongation of the QT interval, drugs that can cause polymorphic ventricular tachycardia of the torsade de pointes type, except for non-antiarrhythmic drugs; inhibition of bone marrow hematopoiesis; reduced circulating blood volume (taking diuretics, salt-free diet, vomiting, diarrhea); coronary heart disease; cerebrovascular diseases; liver and kidney dysfunction; renovascular hypertension; diabetes mellitus; chronic heart failure (functional class IV according to the NYHA classification); hyperuricemia (especially accompanied by gout and urate nephrolithiasis); blood pressure lability; old age; hemodialysis using high-flux membranes (for example, AN69®) or desensitization, LDL apheresis; condition after kidney transplantation; planned anesthesia; Aortic valve stenosis/hypertrophic obstructive cardiomyopathy; atherosclerosis; representatives of the Negroid race (less pronounced effect of use); athletes (possible positive reaction during doping control), bilateral renal artery stenosis or the presence of only one functioning kidney, concomitant therapy with potassium-sparing diuretics, potassium supplements or in patients with elevated plasma potassium levels.
Side Effects
Side Effects
The frequency of side effects is estimated based on: often – 1-10%; rarely – 0.1-1%; extremely rare, including individual reports – less than 0.1%.
From the cardiovascular system: often – excessive decrease in blood pressure and associated symptoms, rarely – arrhythmia, angina pectoris, myocardial infarction and stroke.
From the urinary system: decreased renal function, acute renal failure.
From the respiratory system: often – “dry” cough, difficulty breathing; rarely – bronchospasm, rhinorrhea.
From the digestive system: often – nausea, vomiting, abdominal pain, change in taste, diarrhea or constipation, dry mouth, loss of appetite, cholestatic jaundice, pancreatitis, intestinal edema.
From the central nervous system: often – headache, asthenia, fatigue, dizziness, ringing in the ears, visual disturbances, muscle cramps, paresthesia; rarely – decreased mood, insomnia; extremely rarely – confusion.
Allergic reactions: often – skin rash, itching; rarely – urticaria, angioedema; extremely rarely – exudative erythema multiforme.
Laboratory indicators: often – hypercreatininemia, proteinuria, hyperkalemia; hyperuricemia; rarely (with long-term use in high doses) – neutropenia, leukopenia, hypohemoglobinemia, thrombocytopenia, decreased hematocrit; extremely rarely – agranulocytosis, pancytopenia, increased activity of liver enzymes, hyperbilirubinemia, hemolytic anemia (due to glucose-6-phosphate dehydrogenase deficiency).
Other: increased sweating, impaired sexual function.
Interaction
Interaction
Common to perindopril and indapamide
Combinations not recommended for use
Lithium preparations: with simultaneous use of lithium preparations and ACE inhibitors, a reversible increase in the concentration of lithium in the blood plasma and associated toxic effects have been reported. The simultaneous use of a combination of perindopril and indapamide with lithium preparations is not recommended. If such therapy is necessary, the lithium content in the blood plasma should be regularly monitored.
Combination of drugs requiring special attention and caution
Baclofen: Enhanced antihypertensive effect. Blood pressure should be monitored and, if necessary, the dose of antihypertensive drugs should be adjusted.
Nonsteroidal anti-inflammatory drugs (NSAIDs), including high doses of acetylsalicylic acid (≥ 3 g/day): simultaneous use of ACE inhibitors with NSAIDs (acetylsalicylic acid at a dose that has an anti-inflammatory effect, cyclooxygenase-2 (COX-2) inhibitors and non-selective NSAIDs) may lead to a decrease in the antihypertensive effect. The simultaneous use of ACE inhibitors and NSAIDs may lead to an increased risk of deterioration of renal function, including the development of acute renal failure, and an increase in serum potassium, especially in patients with initially reduced renal function. Caution should be exercised when prescribing a combination of the drug and NSAIDs, especially in elderly patients.
Patients should receive an adequate amount of fluid and it is recommended to monitor renal function both at the beginning of joint therapy and periodically during treatment.
Combination of drugs requiring attention
Tricyclic antidepressants, antipsychotic drugs (neuroleptics): drugs in these classes enhance the antihypertensive effect and increase the risk of orthostatic hypotension (additive effect).
Perindopril
Data from clinical studies show that dual blockade of the RAAS as a result of concomitant use of ACE inhibitors, ARB II or aliskiren leads to an increased incidence of adverse events such as arterial hypotension, hyperkalemia and renal dysfunction (including acute renal failure), compared with situations when only one drug acting on the RAAS is used.
Drugs that cause hyperkalemia
Certain drugs or classes of drugs may increase the incidence of hyperkalemia: aliskiren, potassium salts, potassium-sparing diuretics, ACE inhibitors, ARB II, NSAIDs, heparins, immunosuppressants (such as cyclosporine or tacrolimus), drugs containing trimethoprim, including the fixed combination of trimethoprim and sulfomethoxazole.
The combination of these drugs increases the risk of developing hyperkalemia.
Concomitant use is contraindicated
Aliskiren and medicinal products containing aliskiren
The simultaneous use of ACE inhibitors with medicinal products containing aliskiren is contraindicated in patients with diabetes mellitus and/or moderate or severe renal impairment (GFR < 60 ml/min/1.73 m2 body surface area). The risk of hyperkalemia, deterioration of renal function, cardiovascular morbidity and mortality increases.
Combinations not recommended for use
Aliskiren: In patients without diabetes mellitus or renal impairment, the risk of hyperkalemia, deterioration of renal function and increased incidence of cardiovascular morbidity and mortality is increased.
Combination of therapy with ACE inhibitors and ARB II: According to the available literature, in patients with established atherosclerotic disease, heart failure or diabetes mellitus with target organ damage, the simultaneous use of ACE inhibitors and ARB II leads to an increased incidence of arterial hypotension, syncope, hyperkalemia and renal dysfunction (including acute renal failure), compared with situations where only one drug affecting RA AS. The use of double blockade of RA AS (for example, simultaneous use of an ACE inhibitor and an ARA II) should be limited to isolated cases with strict monitoring of renal function, potassium levels in the blood plasma and blood pressure.
Estramustine: Concomitant use may result in an increased risk of adverse effects such as angioedema.
Potassium-sparing diuretics (eg, triamterene, amiloride) and potassium salts: hyperkalemia (possibly fatal), especially if renal function is impaired (additional effects associated with hyperkalemia).
The combination of perindopril with the above-mentioned drugs is not recommended. However, if concomitant use is indicated, they should be used with caution and regular monitoring of serum potassium levels.
Features of the use of spironolactone in chronic heart failure are described in the subsection “Combination of drugs that require special attention.”
Combination of drugs requiring special attention
Oral hypoglycemic agents and insulin: Epidemiological studies have shown that the combined use of ACE inhibitors and hypoglycemic agents (insulins, oral hypoglycemic agents) may enhance the hypoglycemic effect of insulin and oral hypoglycemic agents, leading to the development of hypoglycemia. This effect is most likely to be observed during the first weeks of concomitant therapy, as well as in patients with impaired renal function.
Non-potassium-sparing diuretics: In patients receiving diuretics, especially those with hypovolemia and/or reduced salt concentrations, an excessive decrease in blood pressure may occur when perindopril therapy is initiated. The risk of developing hypotension can be reduced by discontinuing the diuretic, replacing fluid or salt losses before starting perindopril therapy, as well as prescribing perindopril at a low dose and then gradually increasing it.
For arterial hypertension in patients with hypovolemia or reduced salt concentrations during diuretic therapy, diuretics should either be discontinued before starting the ACE inhibitor (in this case, a potassium-non-sparing diuretic can be reintroduced later), or the ACE inhibitor should be prescribed at a low dose and then gradually increase it.
When using diuretics in the case of chronic heart failure, the ACE inhibitor should be prescribed at a very low dose, possibly after reducing the dose of the concomitantly used potassium-sparing diuretic.
In all cases, renal function (creatinine concentration) should be monitored in the first weeks of using ACE inhibitors.
Potassium-sparing diuretics (eplerenone, spironolactone): use of eplerenone or spironolactone in doses from 12.5 mg to 50 mg per day and low doses of ACE inhibitors:
When treating chronic heart failure of functional class II-IV according to the NYHA classification with a left ventricular ejection fraction <40% and previously used ACE inhibitors and loop diuretics, there is a risk of hyperkalemia (with possible death), especially if the recommendations for this combination of drugs are not followed.
Before using this combination of drugs, you must ensure that there is no hyperkalemia or impaired renal function. It is recommended to regularly monitor the concentration of creatinine and potassium in the blood: weekly in the first month of treatment and monthly thereafter.
mTOR (mammalian target of rapamycin) inhibitors (eg, sirolimus, everolimus, temsirolimus)
Patients receiving concomitant therapy with mTOR inhibitors may have an increased risk of developing angioedema.
Racecadotril
ACE inhibitors (including perindopril) can cause the development of angioedema. The risk of developing angioedema may be increased during concomitant use of racecadotril (an enkephalinase inhibitor used to treat acute diarrhea).
Combination of drugs requiring attention
Antihypertensives and vasodilators: the simultaneous use of these drugs may enhance the antihypertensive effect of perindopril. When administered simultaneously with nitroglycerin, other nitrates or other vasodilators, an additional reduction in blood pressure is possible.
Allopurinol, cytostatic and immunosuppressive drugs, corticosteroids for systemic use and procainamide: simultaneous use with ACE inhibitors may be accompanied by an increased risk of leukopenia.
General anesthetics: ACE inhibitors may enhance the antihypertensive effect of some general anesthetics.
Gliptins (linagliptin, saxagliptin, sitagliptin, vildagliptin): when used together with ACE inhibitors, the risk of angioedema increases due to the inhibition of dipeptidyl peptidase-4 (DPP-IV) activity by gliptin.
Sympathomimetics: may weaken the antihypertensive effect of ACE inhibitors.
Gold preparations: when using ACE inhibitors, including perindopril, nitrite reactions, manifested by facial flushing, nausea, vomiting, and arterial hypotension, have been described in patients receiving intravenous gold preparation (sodium aurothiomalate).
Indapamide
Combination of drugs requiring special attention
Drugs that can cause torsades de pointes: due to the risk of hypokalemia, caution should be exercised when indapamide is used concomitantly with drugs that can cause torsades de pointes, such as class IA (quinidine, hydroquinidine, disopyramide) and class III antiarrhythmics (amiodarone, dofetilide, ibutilide, bretylium, sotalol); some neuroleptics (chlorpromazine, cyamemazine, levomepromazine, thioridazine, trifluoperazine); benzamides (amisulpride, sulpiride, sultopride, tiapride); butyrophenones (droperidol, haloperidol); other antipsychotics (pimozide); other drugs such as bepridil, cisapride, difemanil, erythromycin IV, halofantrine, mizolastine, moxifloxacin, pentamidine, sparfloxacin, vincamine IV, methadone, astemizole, terfenadine. The potassium content in the blood plasma should be monitored and, if necessary, correction should be made; monitor the QT interval.
Drugs that can cause hypokalemia: amphotericin B (iv), gluco- and mineralocorticosteroids (with systemic use), tetracosactide, laxatives that stimulate intestinal motility: increased risk of hypokalemia (additive effect). It is necessary to monitor the potassium content in the blood plasma and, if necessary, correct it. Particular attention should be paid to patients concomitantly receiving cardiac glycosides. Laxatives that do not stimulate intestinal motility should be used.
Cardiac glycosides: hypokalemia enhances the toxic effect of cardiac glycosides. With the simultaneous use of indapamide and cardiac glycosides, the content of potassium in the blood plasma and ECG readings should be monitored and, if necessary, therapy should be adjusted.
Combination of drugs requiring attention
Potassium-sparing diuretics (amiloride, spironolactone, triamterene): this combination is justifiably used in some patients. In this case, hypokalemia or hyperkalemia may occur (especially in patients with renal failure or diabetes mellitus). If simultaneous use of indapamide and potassium-sparing diuretics is necessary, the potassium content in the blood plasma and ECG parameters should be monitored. If necessary, the treatment regimen can be revised.
Metformin: functional renal failure, which can occur while taking diuretics, especially loop diuretics, with simultaneous administration of metformin increases the risk of developing lactic acidosis. Metformin should not be used if the plasma creatinine concentration exceeds 15 mg/l (135 µmol/l) in men and 12 mg/l (110 µmol/l) in women.
Iodinated contrast agents: Dehydration while taking diuretics increases the risk of acute renal failure, especially when using high doses of iodinated contrast agents. Before using iodinated contrast agents, patients need to compensate for fluid loss.
Calcium salts: with simultaneous administration, hypercalcemia may develop due to decreased excretion of calcium ions by the kidneys.
Cyclosporine, tacrolimus: it is possible to increase the concentration of creatinine in the blood plasma without changing the concentration of cyclosporine in the blood plasma, even with normal levels of water and sodium ions.
Corticosteroids, tetracosactide (with systemic use): decreased antihypertensive effect (salt and water retention during the use of corticosteroids).
Overdose
Overdose
Symptoms: marked decrease in blood pressure, nausea, vomiting, muscle cramps, dizziness, drowsiness, confusion, oliguria up to anuria (due to a decrease in blood volume); There may be disturbances in the water-electrolyte balance (low sodium and potassium levels in the blood plasma).
Treatment: gastric lavage and/or administration of activated carbon, restoration of water and electrolyte balance in a hospital setting. If there is a pronounced decrease in blood pressure, it is necessary to transfer the patient to the “lying” position on his back with his legs raised up; then measures should be taken aimed at increasing the volume of blood volume (administration of 0.9% sodium chloride solution intravenously). Perindoprilat, the active metabolite of perindopril, can be removed from the body by dialysis.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 25 ° C.
Keep out of the reach of children.
Shelf life
Shelf life
3 years.
Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
North Star NAO, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date printed on the package. |
|---|---|
| Conditions of storage | In the dark place at a temperature not exceeding 25 °С. Store out of the reach of children. |
| Manufacturer | North Star NAO, Russia |
| Medication form | pills |
| Brand | North Star NAO |
Other forms…
Related products
Buy Perindopril PLUS, tablets 2.5mg+8 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.