No products in the cart.
Pentaflucin, 5 g 10 pcs
€6.37 €5.57
Description
Pentaflucin is a combined medicine with antipyretic and anti-inflammatory effects. Paracetamol has antipyretic and analgesic effects.
A non-narcotic analgesic, blocks COX1 and COX2 mainly in the CNS, affecting the centers of pain and thermoregulation. In inflamed tissues cellular peroxidases neutralize the effect of paracetamol on COX, which explains the almost complete lack of anti-inflammatory effect.
Ascorbic acid plays an important role in the regulation of redox processes, carbohydrate metabolism, blood coagulation, tissue regeneration, increases the body’s resistance.
Diphenhydramine has antihistamine and anti-inflammatory, sedative and hypnotic effect. Calcium gluconate is a regulator of calcium and phosphorus metabolism. Rutosine reduces capillary permeability and fragility, has antioxidant properties.
Indications
Indications
Hyperthermia for colds and other infectious and inflammatory diseases.
Pharmacological effect
Pharmacological effect
Pentaflucin is a combination drug that has antipyretic and anti-inflammatory effects. Paracetamol has an antipyretic and analgesic effect.
A non-narcotic analgesic, it blocks COX1 and COX2 mainly in the central nervous system, affecting the centers of pain and thermoregulation. In inflamed tissues, cellular peroxidases neutralize the effect of paracetamol on COX, which explains the almost complete absence of anti-inflammatory effect.
Ascorbic acid plays an important role in the regulation of redox processes, carbohydrate metabolism, blood clotting, tissue regeneration, and increases the body’s resistance.
Diphenhydramine has antihistamine and anti-inflammatory, sedative and hypnotic effects. Calcium gluconate is a regulator of calcium and phosphorus metabolism. Rutosin reduces the permeability and fragility of capillaries and has antioxidant properties.
Special instructions
Special instructions
To avoid toxic liver damage, you should not combine the drug with drinking alcoholic beverages.
During the treatment period, care must be taken when driving vehicles and engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
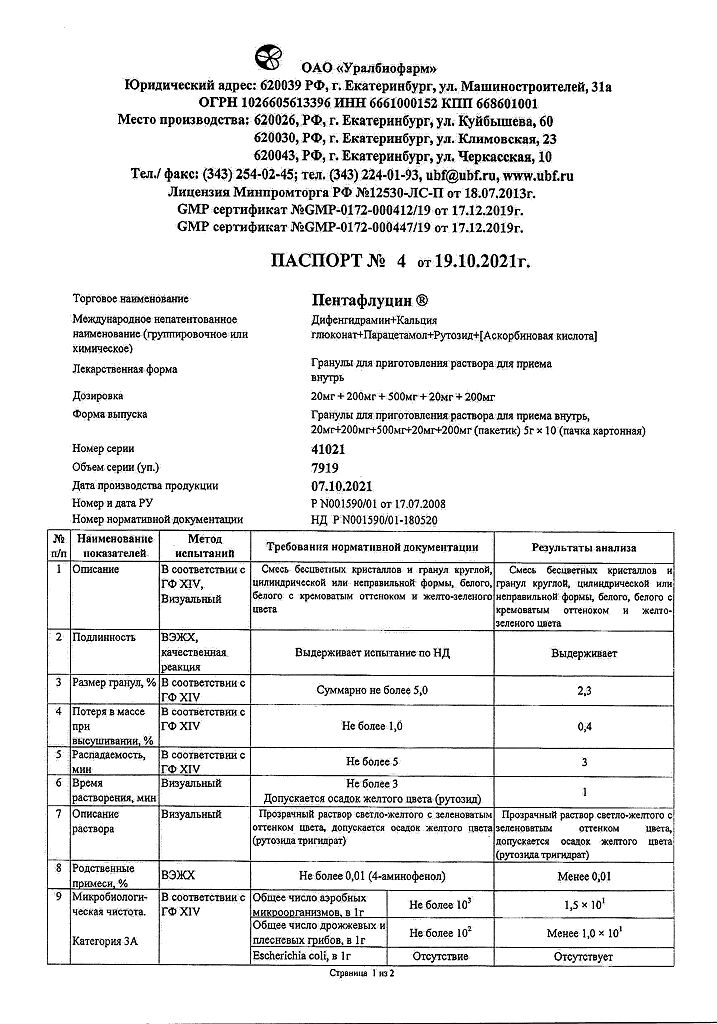
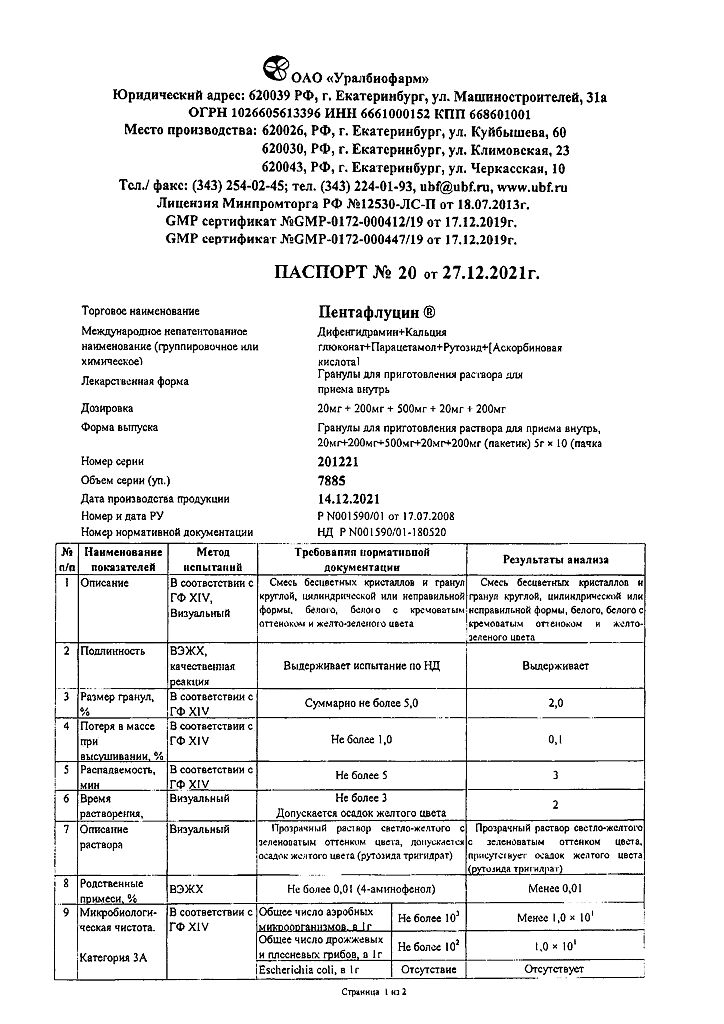
Paracetamol, Ascorbic acid, Rutoside, Diphenhydramine, Calcium gluconate
Composition
Composition
1 sachet (5 g) contains:
Active substances:
ascorbic acid – 0.2 g
calcium gluconate monohydrate – 0.2 g
caffeine (in terms of monohydrate) – 0.02 g
paracetamol – 0.5 g
rutoside trihydrate – 0.02 g
Excipients:
povidone (polyvinylpyrrolidone) – 0.0174 g sucrose (sugar) – 3.9426 g saccharin (soluble saccharin) – 0.050 g citric acid – 0.050 g.
Contraindications
Contraindications
Hypersensitivity to the components of the drug.
Severe liver or kidney dysfunction.
Blood diseases with a tendency to hemorrhage.
Glucose-6-phosphate dehydrogenase deficiency.
Children’s age (up to 18 years).
Congenital fructose intolerance, glucose-galactose malabsorption, sucrose-isomaltase deficiency.
With caution
With Gilbert’s syndrome (constitutional hyperbilirubinemia). The drug contains sugar (1 sachet contains 0.33 XE of sugar), which should be taken into account by patients with diabetes.
Side Effects
Side Effects
At the recommended dosage, adverse reactions are rare. Sometimes allergic skin reactions, nausea, vomiting, headache, general weakness, dizziness may occur.
Ascorbic acid:
From the urinary system: moderate pollakiuria (when taking a dose of more than 600 mg/day), with long-term use of large doses – tiperoxaluria, nephrolithiasis (from calcium oxalate), damage to the glomerular apparatus of the kidneys.
From the digestive system: irritation of the mucous membrane of the gastrointestinal tract.
Allergic reactions: skin rash, skin hyperemia.
Other: inhibition of the function of the pancreatic insular apparatus (hyperglycemia, glycosuria).
Calcium gluconate:
From the digestive system: constipation, irritation of the mucous membrane of the gastrointestinal tract.
Caffeine:
From the nervous system: agitation, anxiety, tremor, restlessness, headache, dizziness, increased reflexes, tachypnea, insomnia. From the cardiovascular system: palpitations, tachycardia, arrhythmias, increased blood pressure.
From the digestive system: nausea, vomiting, exacerbation of peptic ulcer.
Paracetamol:
From the digestive system: nausea, epigastric pain.
From the hematopoietic system: anemia, thrombocytopenia, methemoglobinemia.
Allergic reactions: skin rash, itching, urticaria, angioedema.
With long-term use in high doses, hepatotoxic and nephrotoxic effects are possible.
Rutoside:
Allergic reactions: skin rash
From the digestive system: nausea, diarrhea, heartburn.
From the nervous system: headache.
From the cardiovascular system: “flushes” of blood to the face.
If any of the side effects indicated in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Interaction
Interaction
Combination of the drug with barbiturates, anticonvulsants, phenytoin, carbamazepine, rifampicin, zidovudine and other inducers of microsomal liver enzymes should be avoided.
Paracetamol: reduces the effectiveness of uricosuric drugs. Concomitant use of paracetamol in high doses increases the effect of anticoagulant drugs (decreased synthesis of procoagulant factors in the liver).
Ethanol contributes to the development of acute pancreatitis.
Long-term combined use of paracetamol and other non-steroidal anti-inflammatory drugs increases the risk of developing “analgesic” nephropathy and renal papillary necrosis, and the onset of end-stage renal failure.
Diflunisal increases the plasma concentration of paracetamol by 50% – the risk of developing hepatotoxicity.
Myelotoxic drugs increase the manifestations of hematotoxicity of the drug.
Caffeine: when caffeine is used together with cimetidine, oral contraceptives, ciprofloxacin, norfloxacin – a decrease in the metabolism of caffeine in the liver (slowing its elimination and increasing its concentration in the blood). Mexiletine – reduces caffeine excretion by up to 50%; Nicotine increases the rate of caffeine elimination.
Monoamine oxidase inhibitors, furazolidone, procarbazine and selegiline – large doses of caffeine can cause the development of dangerous cardiac arrhythmias or a marked increase in blood pressure.
Accelerates absorption and enhances the effect of cardiac glycosides, increasing their toxicity.
Concomitant use of caffeine with beta-blockers may lead to mutual suppression of therapeutic effects; with adrenergic bronchodilators – to additional stimulation of the central nervous system and other additive toxic effects.
Caffeine may decrease the clearance of theophylline and possibly other xanthines, increasing the potential for additive pharmacodynamic and toxic effects.
Ascorbic acid: increases the concentration of benzylpenicillin and tetracyclines in the blood.
At a dose of 1 g/day, it increases the bioavailability of ethinyl estradiol (including that contained in oral contraceptives).
Reduces the effectiveness of heparin and indirect anticoagulants. Improves absorption of iron preparations in the intestines (converts ferric iron to divalent iron); may increase iron excretion when used concomitantly with deferoxamine.
Acetylsalicylic acid (ASA), oral contraceptives, fresh juices and alkaline drinks reduce the absorption and absorption of ascorbic acid. ‘
When used simultaneously with ASA, the urinary excretion of ascorbic acid increases and the excretion of ASA decreases. ASA reduces the absorption of ascorbic acid by approximately 30%.
Increases the risk of developing crystalluria during treatment with salicylates and short-acting sulfonamides, slows down the excretion of acids by the kidneys, increases the excretion of drugs that have an alkaline reaction (including alkaloids), and reduces the concentration of oral contraceptives in the blood.
When used simultaneously, it reduces the chronotropic effect of isoprenaline.
Calcium gluconate: when used simultaneously with quinidine, intraventricular conduction may slow down and quinidine toxicity may increase.
Forms insoluble complexes with tetracycline antibiotics (reduces the antibacterial effect).
Slows down the absorption of tetracyclines, digoxin, and oral iron supplements (the interval between doses should be at least 2 hours).
When combined with thiazide diuretics, it can increase hypercalcemia and reduce the effect of calcitonin in hypercalcemia. Reduces the bioavailability of phenytoin.
Pharmaceutically incompatible with carbonates, salicylates, sulfates (forms insoluble or sparingly soluble calcium salts).
Reduces the effect of blockers of “slow” calcium channels.
Rutoside: the pharmacological effect is enhanced by ascorbic acid.
Overdose
Overdose
Symptoms (due to paracetamol): during the first 24 hours after administration – pallor of the skin, nausea, vomiting, anorexia, abdominal pain; impaired glucose metabolism, metabolic acidosis. Symptoms of liver dysfunction may appear 12-48 hours after an overdose. In case of severe overdose – liver failure with progressive encephalopathy, coma, death; acute renal failure with tubular necrosis (including in the absence of severe liver damage); arrhythmia, pancreatitis.
The hepatotoxic effect in adults occurs when taking 10 g or more.
Treatment: the victim should undergo gastric lavage, prescribe adsorbents (activated carbon) and consult a doctor.
Symptomatic therapy: administration of SH-group donors and precursors for the synthesis of glutathione – methionine within 8-9 hours after an overdose and acetylcysteine - within 8 hours. The need for additional therapeutic measures (further administration of methionine, intravenous administration of acetylcysteine) is determined depending on the concentration of paracetamol in the blood, as well as on the time elapsed after its administration.
Storage conditions
Storage conditions
In a dry place, protected from light
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Uralbiopharm, Russia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a dry, light-protected place |
| Manufacturer | Uralbiopharm, Russia |
| Medication form | granules for preparation of oral solution |
| Brand | Uralbiopharm |
Other forms…
Related products
Buy Pentaflucin, 5 g 10 pcs with delivery to USA, UK, Europe and over 120 other countries.