No products in the cart.
Description
Pharmacodynamics
Antitumor drug belonging to a new class of platinum-based compounds in which the platinum atom forms a complex bond with 1,2-diaminocyclohexane (DATG) and oxalate group.
Oxaliplatin has antitumor activity in various types of tumors, including colorectal cancer. It is also effective in the treatment of tumors resistant to cisplatin. The action is manifested irrespective of the phase of the cell cycle. When used with 5-fluorouracil, synergistic cytotoxic action is observed. The mechanism of antitumor effect of oxaliplatin is based on cytotoxic action and is not fully understood. Presumably, oxaliplatin forms inter- and intrathecal bonds with DNA, thus inhibiting its replication and transcription phases.
Pharmacokinetics
In vivo oxaliplatin undergoes active biotransformation and is not detectable in plasma by the end of 2 h after administration at a dose of 130 mg/m2, with 15% of the administered platinum in the blood and the remaining 85% rapidly distributed in the tissues or excreted by the kidneys.
The in vitro biotransformation is the result of non-enzymatic breakdown, and there is no evidence that oxaliplatin is metabolized via cytochrome P450. Oxaliplatin undergoes extensive metabolism, and the drug is not detectable in plasma ultrafiltrate after a 2-hour infusion. Some cytotoxic degradation products of oxaliplatin, including monochloro-, dichloro- and quadro-diaminocyclohexane platinum are detected in plasma along with inactive conjugates later in the study.
Platinum binds to plasma albumin and is excreted in the urine within the first 48 h. By day 5, about 54% of the total dose is detected in the urine and less than 3% in the feces.
Pharmacokinetics in special clinical cases
The effect of renal dysfunction on the distribution of oxaliplatin was studied in patients with varying degrees of renal dysfunction. Oxaliplatin was administered at a dose of 85 mg/m2 in a control group with normal renal function (CK greater than 80 ml/min), in patients with mild (CK 50 to 80 ml/min) and moderate renal function impairment (CK 30 to 49 ml/min); and at a dose of 65 mg/m2 in patients with severe renal impairment (CK less than 30 mL/min). Excretion of oxaliplatin was found to correlate significantly with CK. Renal clearance of platinum was reduced by 30%, 65%, and 84% in patients with mild, moderate, and severe renal impairment, respectively, compared with patients with normal renal function.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
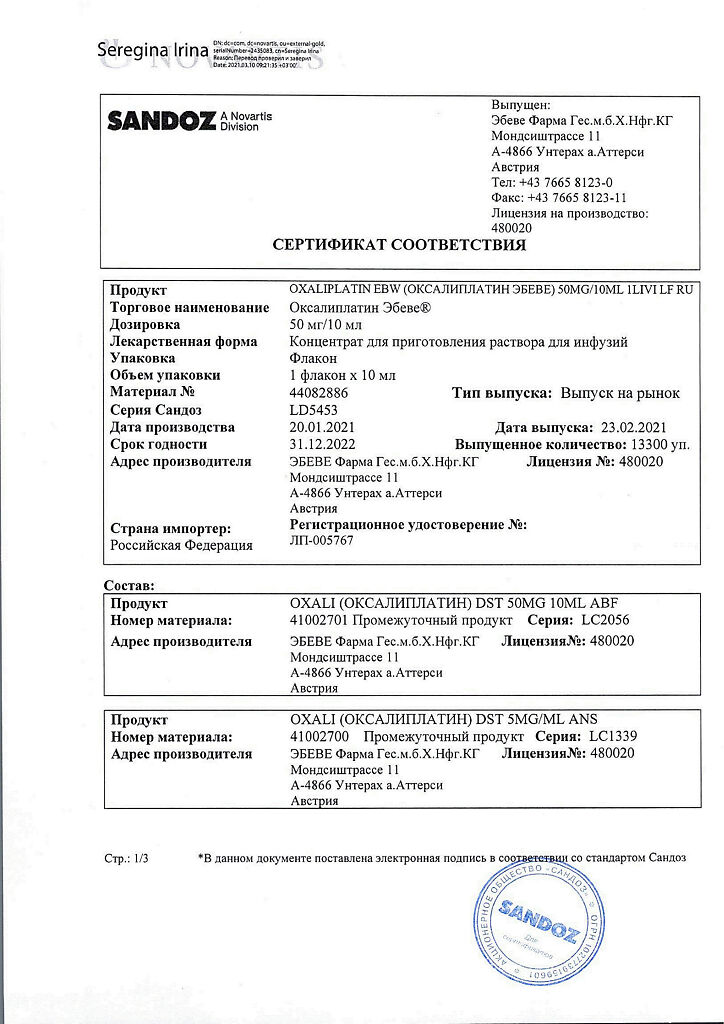
1 vial contains:
The active ingredients:
oxaliplatin 50 mg.
Excipients:
lactose monohydrate.
How to take, the dosage
How to take, the dosage
Oxaliplatin-Ebeve is administered only in adults as an IV infusion for 2-6 hours.
Hyperhydration is not required when using the drug. If oxaliplatin is used in combination with 5-fluorouracil, the infusion of oxaliplatin should precede the administration of 5-fluorouracil.
Adjuvant therapy for colorectal cancer is 85 mg/m2 once every 2 weeks for 12 cycles (6 months).
Treatment of metastatic colorectal cancer – 85 mg/m2 once every 2 weeks as monotherapy or in combination with 5-fluorouracil.
Treatment of ovarian cancer – 85 mg/m2 once every 2 weeks as monotherapy or in combination with other chemotherapeutic agents.
Repeated infusions of Oxaliplatin-Ebeve are given only when neutrophil counts > 1500/μL and platelet counts > 50000/μL.
Recommendations for dosage adjustment and regimen of oxaliplatin administration
If there are hematologic abnormalities (neutrophil count < 1500/μL and/or platelet count < 50000/μL), the next course is delayed until normal laboratory values are restored.
In case of development of diarrhea of grade 4 toxicity (WHO scale), neutropenia of grade 3-4 (neutrophil count < 1000/μl), thrombocytopenia of grade 3-4 (platelet count < 50000/μl) the dose of Oxaliplatin-Ebeve in subsequent administrations should be reduced from 85 mg/msup>2 to 65 mg/m2 for therapy of disseminated colorectal cancer and ovarian cancer; to 75 mg/m2 for adjuvant therapy of colorectal cancer, in addition to the usual dose reduction of 5-fluorouracil when they are combined.
Patients who develop acute laryngeopharyngeal dysesthesia during the infusion or within hours of a 2-hour infusion should have their next Oxaliplatin-Ebeve infusion within 6 hours.
If pain (as a sign of neurotoxicity) persists for more than 7 days or if paresthesia without functional impairment persists until the next cycle, the subsequent dose of Oxaliplatin-Ebeve should be decreased from 85 mg/msup>2 to 65 mg/m2 (for treatment of metastatic cancer) or to 75 mg/m2 (for adjuvant therapy). If paresthesia with functional impairment persists until the next cycle, Oxaliplatin-Ebeve should be withdrawn; if symptoms of neurotoxicity decrease after withdrawal of oxaliplatin, resumption of treatment may be considered.
If stomatitis and/or mucositis of grade 2 or higher toxicity develop, treatment with Oxaliplatin-Ebeve should be suspended until they have resolved or toxicity has been reduced to grade 1.
Patients with impaired renal function
The drug should not be used in patients with significant impairment of renal function. Because of the limited data regarding the safety and tolerability of the drug in patients with moderate renal impairment, the benefit/risk ratio for the patient should be weighed before using the drug. Therapy in this category of patients can be started with the recommended dose, under close monitoring of renal function. No dose adjustment of oxaliplatin is required in mild renal dysfunction.
Patients with impaired hepatic function
There is no need to change the dosing regimen in patients with mild to moderate hepatic impairment. There are no data on the use of oxaliplatin in patients with severe hepatic impairment.
Elderly patients
No dosing adjustment is required when using oxaliplatin in patients over 65 years of age (including when used in combination with 5-fluorouracil).
Regulations for preparation and administration of the solution
Please do not use needles or other equipment containing aluminum when preparing and administering oxaliplatin.
The drug is dissolved in water for injection or in 5% dextrose solution before use, obtaining a solution with a concentration of 5 mg/ml of oxaliplatin (a 50 mg vial takes 10 ml of solvent, a 100 mg vial takes 20 ml of solvent). The drug thus reconstituted is immediately diluted with 250-500 ml of 5% dextrose solution. The concentration of the resulting oxaliplatin solution should be 200 µg/ml to 700 µg/ml; with 700 µg/ml being the highest concentration used in clinical practice at a dose of 85 mg/m2.
Only recommended solvents should be used to prepare the drug solution.
The drug should not be used undiluted.
Do not use 0.9% sodium chloride solution or other saline solutions to dissolve or dilute the drug solution (to prepare infusion solution).
The drug should not be mixed in the same container and should not be administered simultaneously in the same infusion system with other drugs (especially with 5-fluorouracil, alkaline solutions, tromethamol and calcium folinate preparations containing tromethamol in their composition).
Oxaliplatin can be administered together with calcium folinate infusions. In this case, the drugs should not be mixed in the same container for infusion. Calcium folinate for infusion should be diluted using 5% dextrose solution, but under no circumstances should solutions containing sodium chloride or alkaline solutions be used.
The drug solution for infusion is recommended to be used immediately after preparation. The reconstituted solution for infusion remains stable for 24 hours at room temperature (not exceeding 25°C).
The prepared drug solution should be clear and contain no undissolved particles. The solution with signs of precipitation shall be destroyed.
If extravasation occurs, the administration of the drug should be stopped immediately.
Interaction
Interaction
No significant changes in the binding of oxaliplatin to plasma proteins in concomitant use with erythromycin, salicylates, granisetron, paclitaxel and valproic acid were observed.
In interaction with aluminum a precipitate formation and decreased activity of oxaliplatin are possible.
On a single IV infusion of oxaliplatin at a dose of 85 mg/m2, immediately before administration of 5-fluorouracil, no changes in serum concentrations of 5-fluorouracil were observed.
Pharmaceutical interactions
The drug is pharmaceutically incompatible with alkaline solutions and solutions containing chloride.
Do not mix with alkaline drugs or solutions, especially fluorouracil and calcium folinate preparations containing trometamol as an excipient, and with other active substances in the form of trometamol salts.
Special Instructions
Special Instructions
Oxaliplatin-Ebeve should only be used under the supervision of an oncologist experienced in the use of antitumor drugs.
Peripheral blood cells and renal and hepatic function parameters should be monitored regularly (once a week) and before each administration of the drug.
Ahead of each cycle of therapy with Oxaliplatin-Ebeve a neurological examination should be performed to detect signs of neurotoxicity. Patients should be informed about the possibility of persistence of symptoms of peripheral sensory neuropathy after the end of treatment. Localized moderate paresthesias with functional impairment may persist up to 3 years after discontinuation of the drug on adjuvant therapy regimen.
The reversible posterior leukoencephalopathy syndrome (RLS) has been reported in patients receiving oxaliplatin in combination with other chemotherapy drugs. SZOL is a rare, reversible, rapidly developing neurological complication. The main clinical manifestations of SZOL are headache, dizziness, nausea, vomiting, epileptic seizures, behavioral disorders, impaired consciousness (from drowsiness to coma) and visual disturbances in the form of hemianopsia, scotoma, cortical blindness. Since SLE is a potentially life-threatening neurological syndrome and can be complicated by the development of massive brain infarction if not treated in time, its early diagnosis is particularly important, determining the correct treatment of patients. Diagnosis of SLE is based on imaging of the brain by CT or MRI.
If respiratory symptoms occur (dry cough, dyspnea, rales, or pulmonary infiltrates detected on radiologic examination), treatment with Oxaliplatin-Ebeve should be suspended until interstitial pneumonitis is excluded.
In order to prevent and treat gastrointestinal symptoms such as nausea and vomiting, antiemetics are indicated. Symptoms such as dehydration, paralytic ileus, bowel obstruction, hypokalemia, metabolic acidosis and renal failure may be due to severe diarrhea or vomiting, especially when Oxaliplatin-Ebeve is used in combination with 5-fluorouracil.
Patients should be appropriately informed of the risk of diarrhea/vomiting, mucositis/stomatitis and neutropenia when using oxaliplatin and 5-fluorouracil, and should contact their physician if these adverse effects occur for appropriate therapy adjustments.
If liver dysfunction or portal hypertension is detected that is not associated with the presence of liver metastases, drug-induced hepatic vascular dysfunction, specifically the development of hepatic vein obliterating endophlebitis, may occur in very rare cases.
Patients with a history of allergic reactions to other platinum compounds should be monitored for the presence of allergic symptoms.
In case of an anaphylactic-like reaction to Oxaliplatin-Ebeve, the infusion should be stopped immediately and appropriate symptomatic treatment administered. Further use of Oxaliplatin-Ebeve in case of allergic reactions is contraindicated. In case of extravasation the infusion should be stopped immediately and local symptomatic treatment should be started. The remaining dose of the drug should be administered in another vein.
Women and men should use reliable contraceptive methods during treatment and for 6 months after therapy with Oxaliplatin-Ebeve. Because oxaliplatin has a genotoxic effect that may be irreversible, men who wish to have children are advised to consider sperm preservation before starting treatment.
When using Oxaliplatin-Ebeve, all the usual instructions for the use of cytotoxic drugs must be followed. If the drug comes into contact with the skin, immediately rinse the skin thoroughly with soap and water or sodium bicarbonate solution; if it gets into the eyes, pull back the eyelids and rinse the eye (eyes) with plenty of water for 15 minutes.
The remains of the drug and all instruments and materials that have been used to prepare the Oxaliplatin-Ebeve IV infusion solution must be disposed of in accordance with standard hospital procedures for the disposal of cytotoxic waste, subject to current hazardous waste disposal regulations.
The effect of Oxaliplatin-Ebeve on the ability to drive vehicles and other mechanisms requiring high concentration
There have been no studies of the effect of Oxaliplatin-Ebeve on the ability to drive and operate machinery.
Mannered side effects such as dizziness, nausea, vomiting, transient vision loss, other neurologic symptoms may in varying degrees affect the ability to engage in potentially hazardous activities requiring increased concentration and rapid psychomotor reaction.
Contraindications
Contraindications
Side effects
Side effects
According to the WHO, adverse reactions are classified according to their frequency of development as follows:
Overdose
Overdose
Symptoms: myelosuppression, neurotoxicity, diarrhea, nausea, vomiting.
Treatment: hematologic control and symptomatic therapy. An antidote to oxaliplatin is not known.
Additional information
| Weight | 0.039 kg |
|---|---|
| Shelf life | 3 years |
| Conditions of storage | At a temperature not exceeding 25 °C |
| Manufacturer | Fareva Untereh GmbH, Austria |
| Medication form | concentrate for preparation of infusion solution |
| Brand | Fareva Untereh GmbH |
Related products
Buy Oxaliplatin-Ebeve, 50 mg with delivery to USA, UK, Europe and over 120 other countries.