No products in the cart.
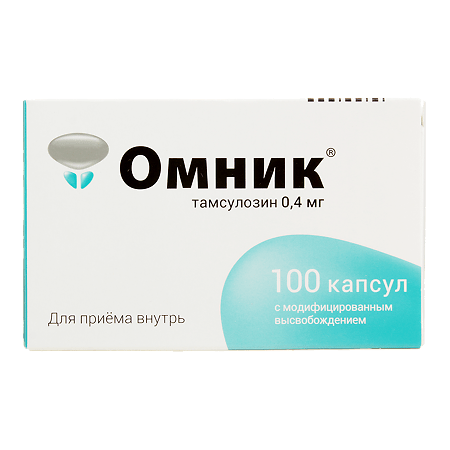
Omnic, 0.4 mg 100 pcs
€63.28 €52.73
Description
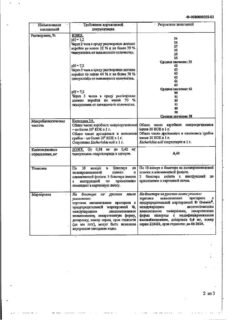
Omnic selectively and competitively blocks postsynaptic α1A-adrenoreceptors located in the smooth muscles of the prostate, bladder neck and prostatic part of the urethra as well as α1D-adrenoreceptors mainly located in the bladder body.
This results in decreasing tone of the smooth muscles of the prostate, bladder neck and prostatic part of the urethra and improving detrusor function. Due to this the symptoms of obstruction and irritation associated with benign prostatic hyperplasia are decreased.
As a rule, therapeutic effect develops within 2 weeks after the beginning of drug intake, although some patients show reduction of symptoms after the first dose.
The ability of Omnik to affect α1A-adrenoreceptors is 20 times greater than its ability to interact with α1B-adrenoreceptors located in the vascular smooth muscle. Due to this high selectivity, the drug does not cause any clinically significant decrease in systemic BP both in patients with arterial hypertension and in patients with normal baseline BP.
Indications
Indications
Treatment of dysuric disorders in benign prostatic hyperplasia.
Pharmacological effect
Pharmacological effect
Omnic selectively and competitively blocks postsynaptic α1A-adrenergic receptors located in the smooth muscles of the prostate gland, bladder neck and prostatic urethra, as well as α1D-adrenergic receptors primarily located in the body of the bladder.
This leads to a decrease in the tone of the smooth muscles of the prostate gland, bladder neck and prostatic urethra and improved detrusor function. This reduces the symptoms of obstruction and irritation associated with benign prostatic hyperplasia.
As a rule, the therapeutic effect develops 2 weeks after starting the drug, although some patients experience a decrease in the severity of symptoms after taking the first dose.
Omnic’s ability to act on α1A-adrenergic receptors is 20 times greater than its ability to interact with α1B-adrenergic receptors, which are located in vascular smooth muscle. Due to such high selectivity, the drug does not cause any clinically significant decrease in systemic blood pressure in both patients with arterial hypertension and in patients with normal baseline blood pressure.
Special instructions
Special instructions
Omnic should be used with caution in patients with a predisposition to orthostatic hypotension. At the first signs of orthostatic hypotension (dizziness, weakness), the patient should be seated or laid down.
Active ingredient
Active ingredient
Tamsulosin
Composition
Composition
1 capsule:
tamsulosin hydrochloride 0.4 mg
excipients:
MCC; methacrylic acid copolymer (type C); polysorbate 80; sodium lauryl sulfate; triacetin; calcium stearate; talc; gelatin; indigotine; titanium dioxide; iron oxide yellow; iron oxide red.
Contraindications
Contraindications
hypersensitivity to tamsulosin or any other component of the drug;
Side Effects
Side Effects
From the cardiovascular system: rarely – dizziness; in isolated cases – orthostatic hypotension, palpitations, tachycardia.
Interaction
Interaction
With simultaneous use of Omnic with cimetidine, a slight increase in the concentration of tamsulosin in the blood plasma was noted, and with furosemide – a decrease in concentration, but this does not require a change in the dose of Omnic.
When used simultaneously with Omnic, diclofenac and warfarin may slightly increase the rate of elimination of tamsulosin. The simultaneous use of Omnic with other alpha1-blockers can lead to a pronounced decrease in blood pressure.
When Omnic was taken simultaneously with atenolol, enalapril, or nifedipine, no drug interactions were detected. Diazepam, propranolol, trichlormethiazide, chlormadinone, amitriptyline, diclofenac, glibenclamide, simvastatin and warfarin do not change the free fraction of tamsulosin in plasma in vitro. In turn, tamsulosin also does not change the free fractions of diazepam, propranolol, trichlormethiazide and chlormadinone.
In vitro studies showed no interaction at the level of hepatic metabolism with amitriptyline, salbutamol, glibenclamide and finasteride.
Overdose
Overdose
There are no reports of cases of acute overdose with tamsulosin. However, theoretically, with an overdose, it is possible to develop an acute decrease in blood pressure and compensatory tachycardia, in which case symptomatic therapy is necessary.
Storage conditions
Storage conditions
The drug should be stored out of the reach of children at a temperature not exceeding 25°C.
Shelf life
Shelf life
4 years
Manufacturer
Manufacturer
ZiO-Zdorovye CJSC, Russia
Additional information
| Shelf life | 4 years |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children at a temperature not exceeding 25°C. |
| Manufacturer | ZiO-Zdorovye CJSC, Russia |
| Medication form | modified-release capsules |
| Brand | ZiO-Zdorovye CJSC |
Other forms…
Related products
Buy Omnic, 0.4 mg 100 pcs with delivery to USA, UK, Europe and over 120 other countries.