No products in the cart.
Octreotide depot, lyophilizate 20 mg
€1.00
Out of stock
(E-mail when Stock is available)
Description
Octreotide depot is a long-acting intramuscular dosage form of Octreotide, which maintains stable therapeutic concentrations of Octreotide in the blood for 4 weeks. Octreotide is a means of pathogenetic therapy for tumors actively expressing receptors to somatostatin. Octreotide is a synthetic octapeptide, which is a derivative of the natural hormone somatostatin and has similar pharmacological effects to it, but much longer duration of action.
The drug suppresses pathologically increased secretion of growth hormone, as well as peptides and serotonin produced in the gastroenteropancreatic endocrine system.
In healthy individuals, octreotide, like somatostatin, suppresses growth hormone secretion induced by arginine, exercise and insulin hypoglycemia; secretion of insulin, glucagon, gastrin and other peptides of the gastroenteropancreatic endocrine system caused by food intake, and insulin and glucagon secretion stimulated by arginine; secretion of thyrotropin caused by thyreoliberin. Suppressive effect on growth hormone secretion in octreotide, unlike somatostatin, is expressed to a much greater extent than on insulin secretion. Administration of octreotide is not accompanied by the phenomenon of hormone hypersecretion by the negative feedback mechanism.
In patients with acromegaly, administration of Octreotide Depot provides in the vast majority of cases a persistent decrease in growth hormone concentration and normalization of insulin-like growth factor 1/somatomedin C (IGF-1) concentration.
In most patients with acromegaly, Octreotide Depot significantly reduces symptoms such as headache, increased sweating, paresthesias, fatigue, bone and joint pain, and peripheral neuropathy. It has been reported that treatment with octreotide in selected patients with pituitary adenomas secreting growth hormone led to a reduction in tumor size.
In carcinoid tumors the use of Octreotide can lead to a reduction in the severity of symptoms of the disease, primarily such as hot flashes and diarrhea. In many cases the clinical improvement is accompanied by a decrease in plasma serotonin concentration and urinary excretion of 5-hydroxyindoleacetic acid.
In tumors characterized by hyperproduction of vasoactive intestinal peptide (VIPoma), use of octreotide leads in most patients to reduction of severe secretory diarrhea, which is characteristic of this condition, which in turn leads to improvement of patient’s quality of life. At the same time there is a reduction of concomitant electrolyte balance disorders, such as hypokalemia, which allows to cancel enteral and parenteral administration of fluids and electrolytes. According to computed tomography data, in some patients there is a slowing or stopping of tumor progression and even reduction of its size, especially of liver metastases. Clinical improvement is usually accompanied by a decrease (down to normal values) in plasma concentration of vasoactive intestinal peptide (VIP).
In glucagonomas, use of Octreotide in most cases results in a marked reduction of the necrotizing migratory rash that is characteristic of this condition. Octreotide has no significant effect on the severity of diabetes mellitus often seen in glucagonomas, and usually does not lead to a reduction in the need for insulin or oral hypoglycemic drugs. In patients with diarrhea, octreotide causes a decrease in diarrhea, which is accompanied by an increase in body weight. When using Octreotide there is often a rapid decrease in plasma glucagon concentration, but with prolonged treatment this effect does not persist. At the same time, symptomatic improvement remains stable for a long time.
In gastrinomas/Zollinger-Ellison syndrome, octreotide used as monotherapy or in combination with H2-histamine receptor blockers and proton pump inhibitors may reduce the formation of hydrochloric acid in the stomach and lead to clinical improvement, including for diarrhea. There may also be a reduction in other symptoms probably related to peptide synthesis by the tumor, including hot flashes. In some cases there is a decrease in plasma concentration of gastrin.
In patients with insulinomas, octreotide decreases the concentration of immunoreactive insulin in blood. In patients with operable tumors Octreotide can provide restoration and maintenance of normoglycemia in the preoperative period. In patients with inoperable benign and malignant tumors, glycemic control may improve without a simultaneous prolonged decrease in blood insulin concentration.
In patients with rare tumors hyperproducing the growth hormone releasing factor (somatoliberinomas), octreotide reduces the severity of acromegaly symptoms. This seems to be due to suppression of growth hormone releasing factor and growth hormone secretion itself. Subsequently, it is possible to reduce the size of the pituitary gland, which was enlarged before the start of treatment.
In patients with hormone-resistant prostate cancer (HRPC), the pool of neuroendocrine cells expressing somatostatin receptors affinity for Octreotide (SS2 and SS5 types) increases, which determines tumor sensitivity to Octreotide. Application of Octreotide-depo in combination with dexamethasone against the background of androgen blockade (medical or surgical castration) in patients with GERD restores sensitivity to hormonal therapy and leads to reduction of prostatic specific antigen (PSA) in more than 50% of patients.
In GRPJ patients with bone metastases, this therapy is accompanied by a pronounced and long-lasting analgesic effect. At the same time, all patients who responded to combined therapy with Octreotide-depo significantly improve quality of life and increase median recurrence-free survival.
Indications
Indications
In the therapy of acromegaly:
In the therapy of endocrine tumors of the gastrointestinal tract and pancreas:
In therapy of hormone-resistant prostate cancer: In combination therapy with surgical or medical castration.
In the prevention of acute postoperative pancreatitis: in extensive abdominal and thoracoabdominal surgical procedures (including for cancer of the stomach, esophagus, colon, pancreas, primary and secondary tumor involvement of the liver).
Active ingredient
Active ingredient
Composition
Composition
Active ingredient:
octreotide;
Associates:
DL-lactic acid and glycolic acid copolymer;
D-mannite;
carboxymethylcellulose sodium salt;
polysorbate-80
How to take, the dosage
How to take, the dosage
V/m, deep into the gluteal muscle. For repeated injections the left and right sides should be alternated. Suspension should be prepared immediately before injection. On the day of injection, the bottle with the drug and the ampoule with the solvent can be kept at room temperature.
In the treatment of acromegaly in patients for whom p/c administration of Octreotide provides adequate control of disease manifestations, the recommended starting dose of Octreotide Depot is 20 mg every 4 weeks for 3 months. Treatment with Octreotide depot may be started the day after the last p/c injection of Octreotide. Subsequently, the dose is adjusted taking into account the serum concentrations of growth hormone and IGF-1 as well as clinical symptoms. If after 3 months of therapy the adequate clinical and biochemical effect was not achieved (in particular, if concentrations of growth hormone are higher than 2.5 µg/L), the dose can be increased up to 30 mg given every 4 weeks.
In cases where, after 3 months of treatment with Octreotide Depot at a dose of 20 mg, there is a steady decrease in serum concentration of growth hormone below 1 µg/L, normalization of IGF-1 concentration and disappearance of reversible acromegaly symptoms, the dose of Octreotide Depot may be reduced to 10 mg. However, in these patients receiving a relatively low dose of Octreotide Depot, serum concentrations of growth hormone and IGF-1 as well as symptoms of the disease should continue to be closely monitored.
Patients receiving a stable dose of Octreotide Depot should have their growth hormone and IGF-1 concentrations determined every 6 months.
Patients in whom surgical treatment and radiation therapy are ineffective or ineffective, as well as patients who require short-term treatment between courses of radiation therapy until its full effect develops, are recommended to have a trial course of treatment with Octreotide injections by injection to evaluate its effectiveness and overall tolerability and only then switch to Octreotide Depot according to the above scheme.
In the treatment of endocrine tumors of the gastrointestinal tract and pancreas in patients for whom pInjection of Octreotide provides adequate control of disease manifestations, the recommended starting dose of Octreotide Depot is 20 mg every 4 weeks. Pc administration of Octreotide should be continued for an additional 2 weeks after the first administration of Octreotide depot.
In patients who have not previously received Octreotide by injection, it is recommended that treatment be initiated with an injection of 0.1 mg of Octreotide three times daily for a relatively short period of time (approximately 2 weeks) to evaluate its effectiveness and overall tolerability. Only after that should Octreotide depot be administered according to the above scheme.
If therapy with Octreotide Depot for 3 months provides adequate control of clinical manifestations and biological markers of the disease, it is possible to reduce the dose of Octreotide Depot to 10 mg administered every 4 weeks.
In cases where only partial improvement has been achieved after 3 months of treatment with Octreotide Depot, the dose may be increased to 30 mg every 4 weeks. During treatment with Octreotide-depo on some days, clinical manifestations characteristic of endocrine tumors of the gastrointestinal tract and pancreas may worsen. In these cases, additional p/c injection of Octreotide at the dose used before the start of treatment with Octreotide-depo is recommended. This may occur primarily during the first 2 months of treatment until therapeutic plasma concentrations of Octreotide are achieved.
In the treatment of GERD, the recommended starting dose of Octreotide Depot is 20 mg every 4 weeks for 3 months. Thereafter, the dose is adjusted according to the dynamics of serum PSA concentrations and clinical symptoms. If after 3 months of treatment it was not possible to achieve adequate clinical and biochemical effect (decrease of PSA), the dose may be increased to 30 mg administered every 4 weeks.
The treatment with Octreotide depot is combined with the use of dexamethasone, which is administered orally according to the following regimen: 4 mg/d for 1 month, then 2 mg/d for 2 weeks, then 1 mg/d (maintenance dose).
The treatment with Octreotide depot and dexamethasone is combined with the use of a GnRH analogue in patients who were previously treated with medication-assisted antiandrogen therapy. In this case the injection of the GnRH analogue (depo-form) is carried out once every 4 weeks.
Patients receiving Octreotide depot should have their PSA concentrations determined every month.
In patients with impaired renal function, hepatic function and elderly patients there is no need to adjust the dosing regimen of Octreotide depot.
In order to prevent acute postoperative pancreatitis, Octreotide Depot in a dose of 10 or 20 mg is administered once no earlier than 5 days and no later than 10 days before the intended surgical intervention.
Regulations for suspension preparation and administration
Interaction
Interaction
Octreotide decreases intestinal absorption of cyclosporine and slows down absorption of cimetidine.
The bioavailability of Octreotide and bromocriptine is increased with concomitant use.
There is literature evidence that somatostatin analogues may decrease the metabolic clearance of substances metabolized by cytochrome P450 enzymes, which may be caused by growth hormone suppression. Because such effects of octreotide cannot be excluded, drugs metabolized by cytochrome P450 enzymes and with a narrow therapeutic range (quinidine and terfenadine) should be prescribed with caution.
Special Instructions
Special Instructions
In pituitary tumors secreting GH, patients must be closely monitored, as tumors may increase in size and develop serious complications such as narrowing of the visual fields. In these cases, the need for other methods of treatment should be considered. In 15-30% of patients treated with Octreotide p/k for a long time, gallstones may occur.
The prevalence in the general population (age 40-60 years) is 5-20%. The experience of long-term treatment with prolonged-acting Octreotide in patients with acromegaly and tumors of the gastrointestinal tract and pancreas shows that prolonged-acting Octreotide, compared to short-acting Octreotide, does not increase the frequency of gallstones formation. However, an ultrasound examination of the gallbladder is recommended before starting treatment with Octreotide depot and approximately every 6 months during treatment.
The gallstones, if detected, are usually asymptomatic. If there are clinical symptoms, conservative treatment (such as the use of bile acids) or surgery is indicated. In patients with type 1 diabetes mellitus, Octreotide Depot may affect glucose metabolism and, therefore, reduce the need for injected insulin. For patients with type 2 diabetes mellitus and patients without concomitant carbohydrate metabolism disorder, p/c injections of Octreotide may result in postprandial glycemia.
In this regard, it is recommended to monitor glycemia levels regularly and, if necessary, to correct hypoglycemic therapy. In patients with insulinomas during treatment with Octreotide an increase in severity and duration of hypoglycemia may be noted (this is due to a more pronounced suppressive effect on GH and glucagon secretion than on insulin secretion as well as a shorter duration of inhibitory effect on insulin secretion).
Systematic follow-up of these patients is indicated. Patients should have an initial ultrasound of the gallbladder before prescribing Octreotide. During treatment with Octreotide depot, repeated ultrasounds of the gallbladder should be performed, preferably at intervals of 6-12 months.
If gallstones are detected before the start of treatment, the potential benefits of therapy with Octreotide Depot should be evaluated against the possible risks associated with the presence of gallstones. There is currently no evidence that Octreotide Depot adversely affects the course or prognosis of pre-existing gallstones.
Contraindications
Contraindications
Hypersensitivity to octreotide or other components of the drug.
With caution: cholelithiasis; diabetes mellitus; pregnancy and lactation.
Side effects
Side effects
Local reactions: when injecting Octreotide-depo intramuscularly, pain, less often – swelling and rash at the injection site are possible (usually mild, short-lived).
Gastrointestinal disorders: anorexia, nausea, vomiting, spastic abdominal pain, abdominal distension, excessive gas, loose stools, diarrhea, steatorrhea. Although excretion of fat with feces may increase, to date there is no evidence that prolonged treatment with Octreotide may lead to the development of deficiency of some nutrients due to impaired absorption (malabsorption). In rare cases, phenomena resembling acute intestinal obstruction may be observed: progressive abdominal bloating, pronounced pain in the epigastric region, abdominal wall tension. Prolonged use of Octreotide-depo may lead to formation of gallstones in the gallbladder.
Pancreatic disorders: rare cases of acute pancreatitis developed during the first hours or days of use of Octreotide have been reported. With long-term use there have been cases of pancreatitis associated with cholelithiasis
Hepatic disorders: there have been isolated reports of the development of liver function disorders (acute hepatitis without cholestasis with normalization of transaminases after discontinuation of Octreotide); slow development of hyperbilirubinemia accompanied by elevation of ALP, GGT, and, to a lesser extent, other transaminases.
In metabolic disorders: since Octreotide Depot has an inhibitory effect on the formation of growth hormone, glucagon and insulin, it may affect glucose metabolism. Glucose tolerance may be decreased after a meal. With long-term use of Octreotidap/k, persistent hyperglycemia may develop in some cases. Hypoglycemic conditions have also been observed.
Others: in rare cases, temporary hair loss after administration of Octreotide, bradycardia, tachycardia, dyspnea, skin rash, anaphylaxis have been reported. There have been isolated reports of the development of hypersensitivity reactions.
Similarities
Similarities
Additional information
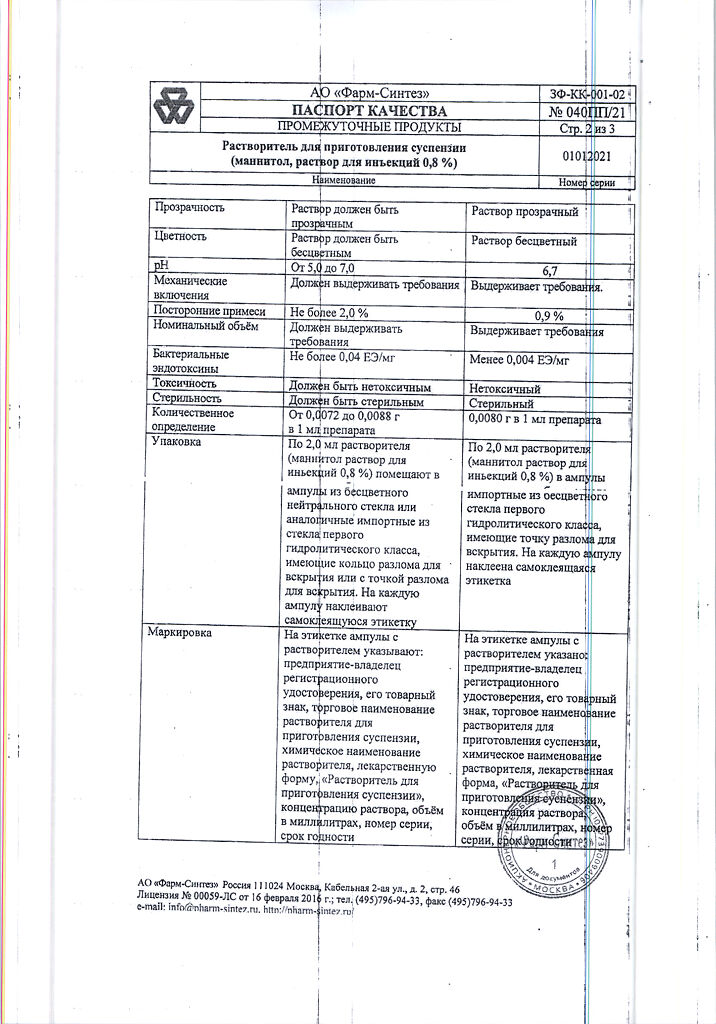
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature of 2 to 8 °C. |
| Manufacturer | Pharm-Sintez, Russia |
| Medication form | lyophilizate |
| Brand | Pharm-Sintez |
Other forms…
Related products
Buy Octreotide depot, lyophilizate 20 mg with delivery to USA, UK, Europe and over 120 other countries.