No products in the cart.
Nurofen Intensive, 200 mg+500 mg 12 pcs
€8.28 €6.90
Description
A combination drug, the action of which is due to its constituent components. It has a directed effect against pain (analgesic), antipyretic and anti-inflammatory effects. Ibuprofen and paracetamol differ in mechanism and site of action. As a result of their mutually reinforcing action a more pronounced reduction of pain sensitivity and increased antipyretic effect is achieved than separately.
Ibuprofen is a derivative of propionic acid from the group of non-steroidal anti-inflammatory drugs (NSAIDs), has anti-inflammatory, anti-edema, analgesic and antipyretic effect. Mechanism of action of ibuprofen is due to inhibition of prostaglandin synthesis – mediators of pain, inflammation and hyperthermia by non-selective inhibition of cyclooxygenase 1 (COX-1) and cyclooxygenase 2 (COX-2) activity.
The analgesic effect of ibuprofen is provided by its inhibitory effect at the peripheral level. Antipyretic effect of ibuprofen is associated with central inhibition of prostaglandin synthesis in hypothalamus. Ibuprofen inhibits migration of leukocytes to the center of inflammation. In addition, ibuprofen reversibly inhibits platelet aggregation.
Paracetamol is an analgesic non-narcotic, has analgesic, antipyretic and mild anti-inflammatory effects. It indiscriminately blocks COX-2, mainly in the central nervous system. Paracetamol can also stimulate the activity of the descending serotonin pathways, which results in knocking out the transmission of the pain impulse in the spinal cord. At peripheral level paracetamol has a weak effect on COX-1 and COX-2.
The drug has a more rapid therapeutic effect and more pronounced analgesic effect than ibuprofen and paracetamol separately. After taking one tablet of the drug the analgesic effect is noted on average after 15 minutes, clinically significant analgesic effect is achieved after 40 minutes and lasts for 8 hours. After taking two tablets of the preparation analgesic effect is noted on the average in 18 minutes, clinically significant analgesic effect is achieved within 45 minutes and lasts for 9 hours.
Pharmacokinetics
Ibuprofen: absorption is high, quickly and almost completely absorbed from the gastrointestinal tract (GIT). The binding to blood plasma proteins is 90%. Slowly penetrates into the joint cavity, lingers in synovial fluid, creating higher concentrations in it than in blood plasma. It is detected in blood plasma 5 minutes after the drug intake on an empty stomach; maximum concentration (Cmax) in blood plasma is reached after 1-2 hours.
Concomitant administration with food may decrease the plasma concentration of ibuprofen and increase the time to reach maximum concentration (Tmax). The degree of absorption of ibuprofen is independent of food intake. It is metabolized in the liver. After absorption, about 60% of the pharmacologically inactive R-form is slowly transformed into the active S-form. The half-life (T1/2) is 2 hours.
Extracted by kidneys mainly as metabolites (unchanged not more than 1 %) and, to a lesser extent, in bile as metabolites. There were no significant differences in the pharmacokinetic profile of ibuprofen in elderly people compared to younger people. There is evidence that ibuprofen was detected in breast milk in insignificant concentrations.
Paracetamol: Absorption is high, quickly absorbed from the gastrointestinal tract. Binding to plasma proteins is insignificant when administered in therapeutic doses; slightly increased in overdose. Detectable in blood plasma 5 minutes after the drug intake on an empty stomach; Cmax in blood plasma is reached 30-40 minutes after intake. Concomitant intake with food may decrease the plasma concentration of paracetamol and increase Tmax. The degree of absorption of paracetamol is independent of food intake.
Metabolized in the liver and excreted mainly as glucuronides and sulfated conjugates with the formation of glutathione conjugates (about 10%). It is excreted by the kidneys.
Less than 5% of the dose taken is excreted as unchanged paracetamol. The elimination half-life (T1/2) is 3 hours. Hydroxylated metabolite N-acetyl-p-benzoquinonimine, which is formed in small amounts in the liver and kidneys under the influence of mixed oxidases and is usually detoxified by binding to glutathione, may accumulate in paracetamol overdose and cause liver tissue damage.
In the elderly there were no significant differences in the pharmacokinetic profile of paracetamol compared to younger people. The bioavailability and pharmacokinetic parameters of ibuprofen and paracetamol taken as part of this combination drug do not change with both single and multiple administration.
Indications
Indications
Back pain, joint pain, muscle and rheumatic pain, neuralgia, headache, migraine, toothache, painful menstruation, sore throat, fever, cold and flu symptoms.
The drug is especially indicated for the symptomatic treatment of pain requiring a more pronounced analgesic effect than ibuprofen or paracetamol alone.
Pharmacological effect
Pharmacological effect
A combined drug whose effect is determined by its constituent components. It has a targeted effect against pain (analgesic), antipyretic and anti-inflammatory effect. Ibuprofen and paracetamol differ in their mechanism and site of action. As a result of their mutually reinforcing action, a more pronounced decrease in pain sensitivity and an increase in antipyretic effect is achieved than individually.
Ibuprofen is a derivative of propionic acid from the group of non-steroidal anti-inflammatory drugs (NSAIDs), has anti-inflammatory, decongestant, analgesic and antipyretic effects. The mechanism of action of ibuprofen is due to inhibition of the synthesis of prostaglandins – mediators of pain, inflammation and hyperthermic reaction, through indiscriminate inhibition of the activity of cyclooxygenase 1 (COX-1) and cyclooxygenase 2 (COX-2).
The analgesic effect of ibuprofen is provided by its inhibitory effect at the peripheral level. The antipyretic effect of ibuprofen is associated with central inhibition of prostaglandin synthesis in the hypothalamus. Ibuprofen inhibits the migration of leukocytes to the site of inflammation. In addition, ibuprofen reversibly inhibits platelet aggregation.
Paracetamol is an analgesic non-narcotic drug that has analgesic, antipyretic and weak anti-inflammatory effects. Indiscriminately blocks COX-2, mainly in the central nervous system. Paracetamol can also stimulate the activity of the descending serotonin pathways, which leads to the inhibition of the transmission of pain impulses in the spinal cord. At the peripheral level, paracetamol has a weak effect on COX-1 and COX-2.
The drug has a faster therapeutic effect and a more pronounced analgesic effect than ibuprofen and paracetamol separately. After taking one tablet of the drug, the analgesic effect is observed on average after 15 minutes, a clinically significant analgesic effect is achieved after 40 minutes and lasts for 8 hours. After taking two tablets of the drug, the analgesic effect is observed after an average of 18 minutes, a clinically significant analgesic effect is achieved after 45 minutes and lasts for 9 hours.
Pharmacokinetics
Ibuprofen: absorption is high, quickly and almost completely absorbed from the gastrointestinal tract (GIT). Connection with blood plasma proteins – 90%. Slowly penetrates into the joint cavity, lingers in the synovial fluid, creating higher concentrations in it than in the blood plasma. Detected in the blood plasma 5 minutes after taking the drug on an empty stomach, the maximum concentration (Cmax) in the blood plasma is achieved after 1-2 hours.
Concomitant use with food may reduce the plasma concentration of ibuprofen and increase the time to reach maximum concentration (Tmax). The degree of absorption of ibuprofen does not depend on food intake. Metabolized in the liver. After absorption, about 60% of the pharmacologically inactive R-form is slowly transformed into the active S-form. The half-life (T1/2) is 2 hours.
It is excreted by the kidneys mainly in the form of metabolites (no more than 1% unchanged) and, to a lesser extent, with bile in the form of metabolites. There were no significant differences in the pharmacokinetic profile of ibuprofen in older adults compared to younger adults. There is evidence that ibuprofen was found in breast milk in trace concentrations.
Paracetamol: high absorption, quickly absorbed from the gastrointestinal tract. Communication with plasma proteins is insignificant when taken in therapeutic doses; increases slightly with overdose. Detected in the blood plasma 5 minutes after taking the drug on an empty stomach, Cmax in the blood plasma is achieved 30-40 minutes after administration. Concomitant use with food can reduce the concentration of paracetamol in the blood plasma and increase Tmax. The degree of absorption of paracetamol does not depend on food intake.
Metabolized in the liver and excreted mainly in the form of glucuronides and sulfated conjugates with the formation of glutathione conjugates (about 10%). Excreted by the kidneys.
Less than 5% of the dose taken is excreted as unchanged paracetamol. The half-life (T1/2) is 3 hours. The hydroxylated metabolite N-acetyl-p-benzoquinoneimine, which is formed in trace amounts in the liver and kidneys by mixed oxidases and is usually detoxified by binding to glutathione, can accumulate during paracetamol overdose and cause liver tissue damage.
There were no significant differences in the pharmacokinetic profile of paracetamol in older adults compared to younger adults. The bioavailability and pharmacokinetic parameters of ibuprofen and paracetamol taken as part of this combination drug do not change with either single or repeated use.
Special instructions
Special instructions
It is recommended to take the drug for the shortest possible course and in the minimum effective dose necessary to eliminate symptoms.
In patients with bronchial asthma or an allergic disease in the acute stage, as well as in patients with a history of bronchial asthma/allergic disease, the drug may provoke bronchospasm.
Use of the drug in patients with systemic lupus erythematosus or mixed connective tissue disease is associated with an increased risk of developing aseptic meningitis.
During long-term treatment, monitoring of the peripheral blood picture and the functional state of the liver and kidneys is necessary. When symptoms of gastropathy appear, careful monitoring is indicated, including esophagogastroduodenoscopy, a complete blood count (hemoglobin determination), and a stool test for occult blood. If it is necessary to determine 17-ketosteroids, the drug should be discontinued 48 hours before the study.
During the treatment period, ethanol intake is not recommended.
Patients with renal failure should consult a doctor before using the drug, as there is a risk of deterioration in the functional state of the kidneys.
Patients with hypertension, including a history of hypertension and/or chronic heart failure, should consult a physician before using the drug, as the drug may cause fluid retention, increased blood pressure and edema.
In patients with uncontrolled blood pressure, NYHA class II-III congestive heart failure, coronary artery disease, peripheral arterial disease and/or cerebrovascular disease, ibuprofen should be prescribed only after a careful benefit/risk assessment, and high doses of ibuprofen (≥2400 mg/day) should be avoided.
The use of NSAIDs in patients with chickenpox may be associated with an increased risk of developing severe purulent complications of infectious and inflammatory diseases of the skin and subcutaneous fat (for example, necrotizing fasciitis). In this regard, it is recommended to avoid using the drug for chickenpox.
Information for women planning pregnancy: these drugs suppress cyclooxygenase and prostaglandin synthesis, affect ovulation, disrupting female reproductive function (reversible after discontinuation of treatment).
Should not be taken with any other medicines containing paracetamol. If this happens, you must urgently seek medical help, even if you feel well, because… this may lead to an overdose.
Impact on the ability to drive vehicles and machinery
If the recommended dosage regimen and timing of use are followed, the drug does not affect the ability to drive vehicles and machinery, as well as engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
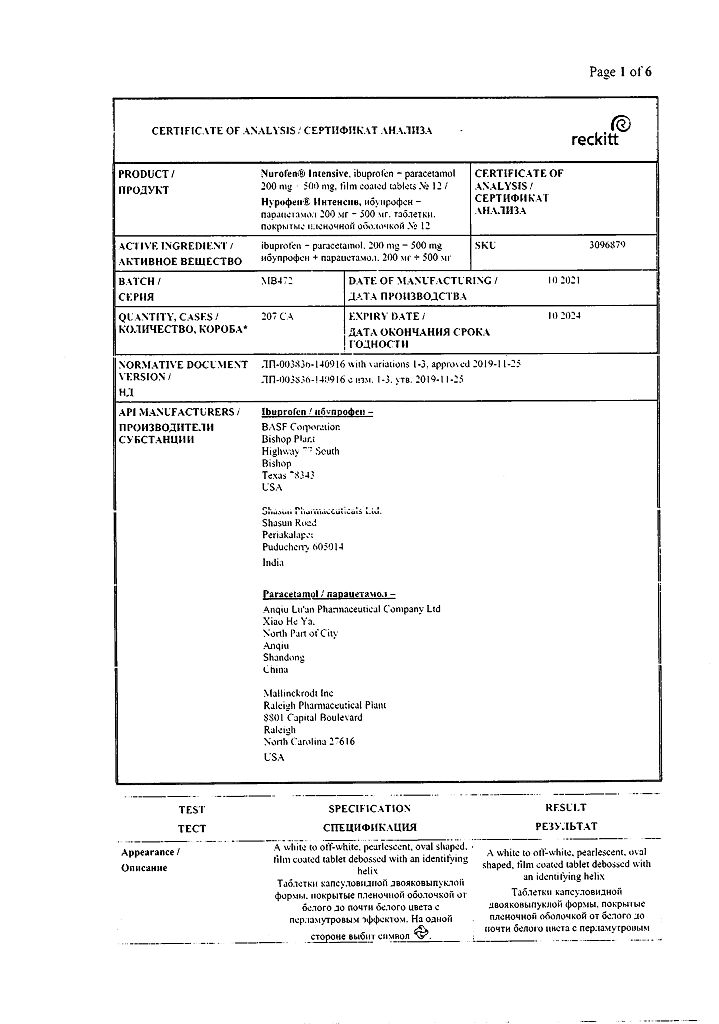
Active ingredient
Active ingredient
Ibuprofen, Paracetamol
Composition
Composition
One film-coated tablet contains:
active ingredients:
ibuprofen 200 mg and paracetamol 500 mg;
excipients:
croscarmellose sodium 30 mg,
microcrystalline cellulose 120 mg,
colloidal silicon dioxide 3 mg,
magnesium stearate 5 mg,
stearic acid 4 mg.
Shell composition:
white film shell 13 mg (polyvinyl alcohol 40%, titanium dioxide 25%, macrogol 20.2%, talc 14.8%), film shell with pearlescent effect 7 mg (polyvinyl alcohol 47%, talc 27%, macrogol 13.3%, mica-based pearlescent pigment 10% (titanium dioxide 28%, potassium aluminosilicate (E555) 72%), polysorbate 2.7%).
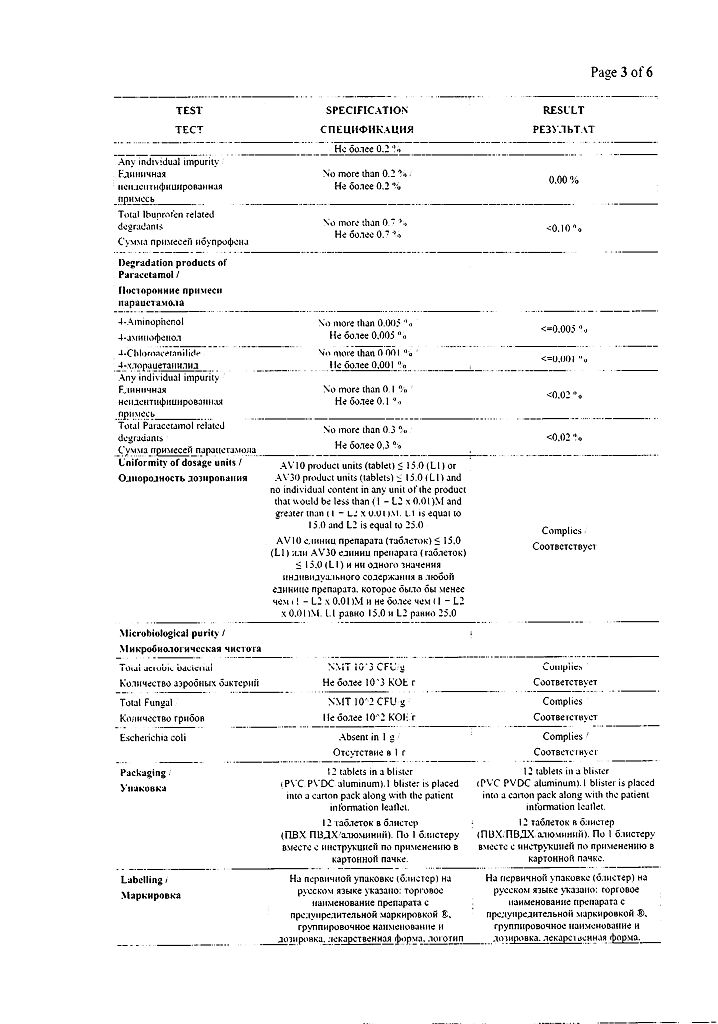
Contraindications
Contraindications
· Hypersensitivity to ibuprofen, paracetamol or other components of the drug.
· Concomitant use of other medications containing paracetamol.
· Complete or incomplete combination of bronchial asthma, recurrent polyposis of the nose and paranasal sinuses, and intolerance to acetylsalicylic acid or other NSAIDs (including a history).
· Erosive and ulcerative diseases of the gastrointestinal tract (including gastric and duodenal ulcers, Crohn’s disease, ulcerative colitis) or ulcerative bleeding in the active phase or in history (two or more confirmed episodes of peptic ulcer or ulcerative bleeding).
· A history of bleeding or perforation of a gastrointestinal ulcer caused by the use of NSAIDs.
· Severe heart failure (NYHA class IV – classification of the New York Heart Association).
· Severe liver failure or active liver disease.
· Severe renal failure (creatinine clearance
<30 ml/min), confirmed hyperkalemia.
· Decompensated heart failure; period after coronary artery bypass surgery.
· Cerebrovascular or other bleeding.
· Pregnancy (III trimester).
· Age up to 18 years.
· Hemophilia and other bleeding disorders (including hypocoagulation), hemorrhagic diathesis.
· Genetic absence of glucose-6-phosphate dehydrogenase.
With caution
If you have the conditions listed in this section, you should consult a doctor before using the drug.
Concomitant use of other NSAIDs, a history of a single episode of gastric ulcer or gastrointestinal ulcer bleeding; gastritis, enteritis, colitis, the presence of Helicobacter pylori infection, ulcerative colitis; bronchial asthma or allergic diseases in the acute stage or in history – bronchospasm may develop; systemic lupus erythematosus or mixed connective tissue disease (Sharpe’s syndrome) – increased risk of aseptic meningitis; chicken pox; renal failure, including dehydration (creatinine clearance less than 30-60 ml/min); nephrotic syndrome; liver failure; frequent drinking of alcohol; arterial hypertension and/or heart failure, cerebrovascular diseases; simultaneous use of drugs that may increase the risk of ulcers or bleeding, in particular oral glucocorticosteroids (including prednisolone), anticoagulants (including warfarin), selective serotonin reuptake inhibitors (including citalopram, fluoxetine, paroxetine, sertraline) or antiplatelet agents (including acetylsalicylic acid, clopidogrel); pregnancy I-II trimester, breastfeeding period; old age; liver cirrhosis with portal hypertension, hyperbilirubinemia; blood diseases of unknown etiology (leukopenia and anemia), hyperlipidemia, diabetes mellitus, peripheral arterial diseases.
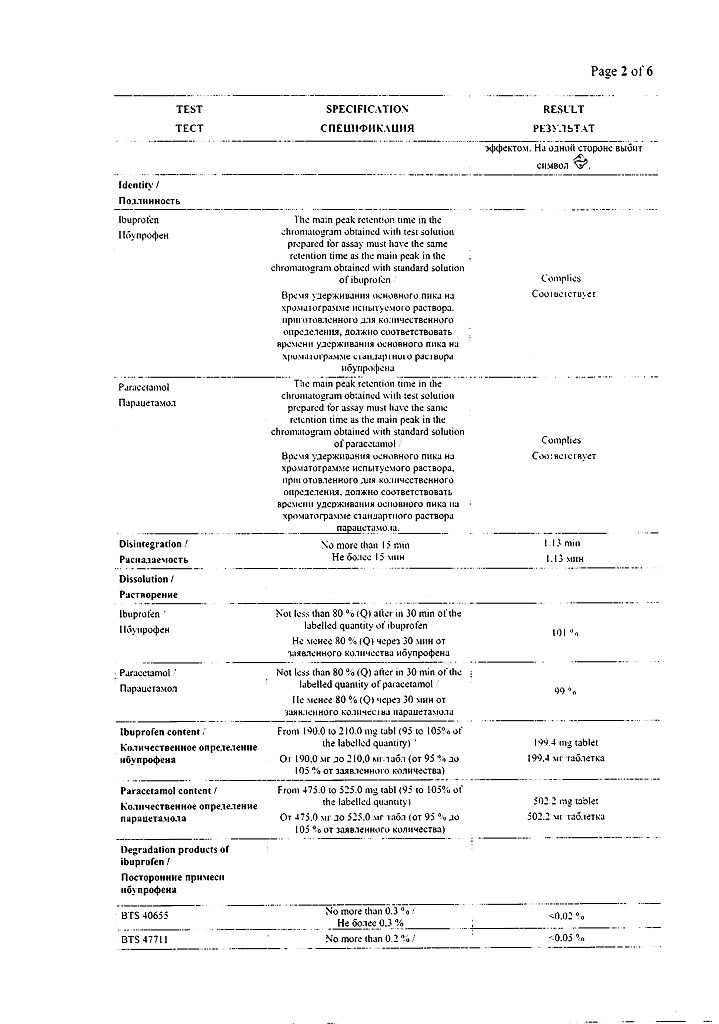
Side Effects
Side Effects
The risk of side effects can be minimized if the drug is taken in a short course, at the minimum effective dose required to eliminate symptoms.
Elderly people experience an increased incidence of adverse reactions with NSAID use, especially gastrointestinal bleeding and perforation, in some cases fatal.
Side effects are predominantly dose-dependent. In particular, the risk of gastrointestinal bleeding depends on the dose range and duration of treatment.
The following adverse reactions were observed with short-term use of ibuprofen in doses of 1200 mg/day, paracetamol in doses of 3000 mg/day (6 tablets). When treating chronic conditions and with long-term use, other side effects may occur.
The incidence of adverse reactions was assessed based on the following criteria: very common (≥ 1/10), common (from ≥ 1/100 to < 1/10), uncommon (from ≥ 1/1000 to < 1/100), rare (from ≥ 1/10,000 to < 1/1000), very rare (≤ 1/10 000), frequency unknown (no frequency estimates available).
Blood and lymphatic system disorders:
Very rare: hematopoietic disorders (anemia, leukopenia, aplastic anemia, hemolytic anemia, thrombocytopenia, pancytopenia, agranulocytosis). The first symptoms of such disorders are fever, sore throat, superficial mouth ulcers, flu-like symptoms, severe weakness, nosebleeds and subcutaneous hemorrhages, bleeding and bruising of unknown origin.
Immune system disorders:
Uncommon: hypersensitivity reactions – nonspecific allergic reactions and anaphylactic reactions, skin reactions (itching, urticaria), allergic rhinitis, eosinophilia.
Very rare: severe hypersensitivity reactions including swelling of the face, tongue and larynx, shortness of breath, tachycardia, low blood pressure (anaphylaxis, angioedema or severe anaphylactic shock).
Nervous system disorders:
Uncommon: headache.
Very rare: aseptic meningitis. In patients with autoimmune disorders such as systemic lupus erythematosus, mixed connective tissue disease, during treatment with ibuprofen, isolated cases of symptoms of aseptic meningitis were observed: stiff neck, headache, nausea, vomiting, fever and disorientation, confusion, depression, hallucinations.
Cardiovascular system disorders:
Frequency unknown: heart failure, peripheral edema, with long-term use there is an increased risk of thrombotic complications (for example, myocardial infarction), increased blood pressure.
Disorders of the respiratory system, chest and mediastinal organs:
Frequency unknown: bronchial asthma, including its exacerbation, bronchospasm, shortness of breath.
Gastrointestinal disorders:
Uncommon: abdominal pain, nausea, dyspepsia (including heartburn, bloating).
Rare: diarrhea, flatulence, constipation, vomiting.
Very rare: peptic ulcer, perforation or gastrointestinal bleeding, melena, hematemesis, in some cases fatal, especially in elderly patients, ulcerative stomatitis, gastritis.
Frequency unknown: exacerbation of colitis and Crohn’s disease.
Disorders of the liver and biliary tract:
Very rare: liver dysfunction (especially with long-term use), increased activity of liver transaminases, hepatitis and jaundice.
Renal and urinary tract disorders:
Very rare: acute renal failure (compensated and decompensated), especially with long-term use, in combination with an increase in the concentration of urea in the blood plasma and the appearance of edema, papillary necrosis, hematuria and proteinuria, nephritic syndrome, nephrotic syndrome, interstitial nephritis, cystitis.
Disorders of the skin and subcutaneous tissues:
Common: hyperhidrosis (excessive sweating).
Uncommon: Skin rash and acute generalized exanthematous pustulosis.
Very rare: Exfoliative and bullous dermatoses, including toxic epidermal necrolysis (Lyell’s syndrome), Stevens-Johnson syndrome, erythema multiforme.
Laboratory indicators:
Frequent: increased levels of alanine aminotransferase, gamma-glutamyl transpeptidase, increased plasma concentrations of creatinine and urea, liver function tests outside the normal range.
Uncommon: increased levels of aspartate aminotransferase, alkaline phosphatase, creatinine phosphokinase, decreased hemoglobin levels, increased platelet levels.
If side effects occur, you should stop taking the drug and consult a doctor.
Interaction
Interaction
Paracetamol
· Antiemetics: decreased rate of absorption of paracetamol when used simultaneously with metoclopramide or domperidone.
· Anticoagulants: long-term use of drugs containing paracetamol may enhance the effect of anticoagulants, in particular warfarin, and increase the risk of bleeding.
· Cholestyramine: decreased rate of absorption of paracetamol when used simultaneously with cholestyramine.
Paracetamol falsely increases continuous blood sugar monitoring (CGM) readings compared to blood glucose meter readings. This applies to patients using CGM devices that do or do not contain an automatic insulin pump, i.e. with type I diabetes.
Caution should be exercised when using paracetamol and flucloxacillin simultaneously, which is associated with an increased risk of developing metabolic acidosis with a high anion gap, especially in patients with a risk factor for developing glutathione deficiency (including patients with severe renal failure, sepsis, malnutrition and chronic alcoholism).
Close monitoring is recommended to identify signs of acid-base imbalance, namely metabolic acidosis with a high anion gap, including the determination of 5-oxoproline in the urine.
Ibuprofen
The simultaneous use of ibuprofen with the following drugs should be avoided:
· Acetylsalicylic acid: with the exception of low doses of acetylsalicylic acid (no more than 75 mg per day) prescribed by a doctor, since combined use may increase the risk of side effects. With simultaneous use, ibuprofen reduces the anti-inflammatory and antiplatelet effect of acetylsalicylic acid (an increase in the incidence of acute coronary insufficiency in patients receiving small doses of acetylsalicylic acid as an antiplatelet agent is possible after starting ibuprofen).
· Other NSAIDs, in particular selective COX-2 inhibitors: the simultaneous use of two or more drugs from the NSAID group should be avoided due to a possible increased risk of side effects.
Use with caution simultaneously with the following medications:
· Anticoagulants and thrombolytic drugs: NSAIDs may enhance the effect of anticoagulants, in particular warfarin and thrombolytic drugs.
· Antihypertensive drugs (ACE inhibitors and angiotensin II antagonists) and diuretics: NSAIDs may reduce the effectiveness of drugs in these groups. In some patients with impaired renal function (eg, dehydrated patients or elderly patients with impaired renal function), co-administration of ACE inhibitors or angiotensin II antagonists and cyclooxygenase inhibitors may lead to a deterioration of renal function, including the development of acute renal failure (usually reversible). These interactions should be considered in patients taking coxibs concomitantly with ACE inhibitors or angiotensin II antagonists. In this regard, the combined use of the above drugs should be prescribed with caution, especially in the elderly. It is necessary to prevent dehydration in patients, and consider monitoring renal function after initiation of this combination treatment and periodically thereafter. Diuretics and ACE inhibitors may increase the nephrotoxicity of NSAIDs.
· Glucocorticosteroids: increased risk of gastrointestinal ulceration and gastrointestinal bleeding.
· Antiplatelet agents and selective serotonin reuptake inhibitors: increased risk of gastrointestinal bleeding.
· Cardiac glycosides: simultaneous administration of NSAIDs and cardiac glycosides can lead to worsening heart failure, a decrease in glomerular filtration rate and an increase in the concentration of cardiac glycosides in the blood plasma.
· Lithium preparations: there is evidence of the likelihood of an increase in the concentration of lithium in the blood plasma during the use of NSAIDs.
· Methotrexate: there is evidence of the likelihood of an increase in the concentration of methotrexate in the blood plasma during the use of NSAIDs.
· Cyclosporine: increased risk of nephrotoxicity with simultaneous administration of NSAIDs and cyclosporine.
· Mifepristone: NSAIDs should be started no earlier than 8-12 days after taking mifepristone, as NSAIDs may reduce the effectiveness of mifepristone.
· Tacrolimus: When NSAIDs and tacrolimus are co-administered, the risk of nephrotoxicity may increase.
· Zidovudine: Concomitant use of NSAIDs and zidovudine may result in increased hematotoxicity. There is evidence of an increased risk of hemarthrosis and hematomas in HIV-positive patients with hemophilia who received concomitant treatment with zidovudine and ibuprofen.
· Quinolone antibiotics: In patients receiving concomitant treatment with NSAIDs and quinolone antibiotics, the risk of seizures may be increased.
· Myelotoxic drugs increase the manifestations of hematotoxicity of the drug.
· Caffeine enhances the analgesic effect.
Overdose
Overdose
Paracetamol
Symptoms: Symptoms of paracetamol overdose during the first 24 hours: pale skin, nausea, vomiting, anorexia and abdominal pain. Liver damage may occur 12 to 48 hours after ingestion, so you should consult a doctor even if you have no symptoms. Impaired glucose metabolism and metabolic acidosis are possible.
In severe poisoning, liver failure can progress with complications such as encephalopathy, hemorrhage, hypoglycemia, cerebral edema and death. Acute renal failure with acute tubular necrosis (defined by low back pain, hematuria, and proteinuria) can develop even in the absence of severe liver damage. There have been reports of cardiac arrhythmias and pancreatitis.
Treatment: Immediate treatment is necessary for paracetamol overdose. Despite the lack of significant early symptoms, patients should be rushed to hospital for immediate medical examination. Symptoms may be limited to nausea or vomiting and may not be consistent with
severity of overdose or risk of organ damage. Treatment with activated charcoal should be considered if the excessive dose was taken less than 1 hour ago. Plasma concentrations of paracetamol should be measured 4 hours or more after dosing (earlier concentrations are unreliable). Treatment with N-acetylcysteine can be carried out up to 24 hours after taking paracetamol, but the maximum protective effect is achieved about 8 hours after taking the drug.
The effectiveness of the antidote gradually decreases after this time. If necessary, N-acetylcysteine is administered intravenously in accordance with the established dosage regimen. Outside the hospital, if there is no vomiting, methionine can be used orally. Patients presenting with severe hepatic dysfunction 24 hours after taking the drug should be referred to a poisoning specialist.
Additional information on special patient groups
An increased risk of liver damage from paracetamol overdose is most likely in:
· patients receiving long-term treatment with enzyme-inducing drugs (such as carbamazepine, phenobarbitone, phenytoin, primidone, rifampicin and St. John’s wort);
· patients who drink alcohol in quantities higher than recommended;
· patients with glutathione depletion (for example, patients with eating disorders, cystic fibrosis, HIV infection, cachexia, fasting).
Ibuprofen
In adults, the dose-dependent effect does not have a clear threshold. The half-life of the drug in case of overdose is 1.5-3 hours.
Symptoms: nausea, vomiting, epigastric pain or, less commonly, diarrhea, tinnitus, headache and gastrointestinal bleeding. In more severe cases, manifestations from the central nervous system are observed: drowsiness, rarely – agitation, convulsions, disorientation, coma.
In cases of severe poisoning, metabolic acidosis and increased prothrombin time, renal failure, liver tissue damage, decreased blood pressure, respiratory depression and cyanosis may develop. In patients with bronchial asthma, exacerbation of this disease is possible.
Treatment: symptomatic, with mandatory maintenance of airway patency, monitoring of ECG and vital signs until the patient’s condition is normalized. Oral use of activated charcoal or gastric lavage is recommended within 1 hour after taking a potentially toxic dose of ibuprofen.
If ibuprofen has already been absorbed, an alkaline drink may be prescribed in order to eliminate the acidic derivative of ibuprofen by the kidneys, forced diuresis. Frequent or prolonged seizures should be treated with intravenous diazepam or lorazepam. If bronchial asthma worsens, the use of bronchodilators is recommended.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25 °C.
Shelf life
Shelf life
3 years.
Manufacturer
Manufacturer
Reckitt Benckiser Healthcare International Ltd, UK
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25 ° C. |
| Manufacturer | Reckitt Benckiser Healthcare International Ltd, United Kingdom |
| Medication form | pills |
| Brand | Reckitt Benckiser Healthcare International Ltd |
Other forms…
Related products
Buy Nurofen Intensive, 200 mg+500 mg 12 pcs with delivery to USA, UK, Europe and over 120 other countries.