No products in the cart.
Description
Anti-allergic agent – H1-histamine receptor blocker.
Pharmacodynamics:
Bilastin is a long-acting antihistamine agent that selectively blocks peripheral H1-receptors.
The significant therapeutic effect is observed one hour after taking the drug, the antihistamine effect lasts for 24 hours.
Possible minor penetration of bilastin through the blood-brain barrier, but bilastin has no significant effect on the central nervous system and does not cause sedation.
It has no anticholinergic effect. No prolongation of the QT interval on the ECG is observed.
Pharmacokinetics:
Intake. After oral administration, bilastin is rapidly absorbed from the gastrointestinal tract. Time of reaching maximum blood plasma concentration (TSmax) is 1.3 h. Bioavailability of bilastin when taken orally is 61%. Concomitant intake of food reduces the bioavailability of bilastin by 30%. No drug accumulation is observed. Binding to plasma proteins is 84-90%.
Metabolism and excretion. Bilastin is mildly metabolized, after a single use up to 95% of the administered dose is excreted unchanged by the kidneys (28.3%) and with the bile (66.5%). The elimination half-life (T1/2) is on average 14.5 hours.
In moderate renal failure (glomerular filtration rate (GFR) 30-50 ml/min/1.73 m2) and severe renal failure (GFR < 30 ml/min/1.73 m2) the elimination rate of bilastine is slowed, which may lead to increased plasma bilastine concentration.
Changing pharmacokinetic parameters has no effect on the safety profile of bilastin, since the plasma concentration of bilastin in patients with renal impairment remains within tolerance.
In hepatic insufficiency there are no clinically significant changes in pharmacokinetic parameters of bilastin since bilastin is slightly metabolized in the liver.
The pharmacokinetic parameters of bilastin in elderly patients are similar to those in younger patients.
Indications
Indications
Allergic (seasonal and year-round) rhinoconjunctivitis: elimination or relief of symptoms (sneezing, feeling of nasal congestion, itching of the nasal mucosa, rhinorrhea, burning and itching sensation in the eyes, redness of the eyes, lacrimation);
urticaria: elimination or reduction of skin itching, rash.
Pharmacological effect
Pharmacological effect
Antiallergic agent – H1-histamine receptor blocker.
Pharmacodynamics:
Bilastine is a long-acting antihistamine that selectively blocks peripheral H1 receptors.
A significant therapeutic effect is observed one hour after taking the drug, the antihistamine effect persists for 24 hours.
A slight penetration of bilastine through the blood-brain barrier is possible, but bilastine does not have a significant effect on the central nervous system and does not cause a sedative effect.
Does not have anticholinergic effect. There is no prolongation of the QT interval on the ECG.
Pharmacokinetics:
Suction. After oral administration, bilastine is rapidly absorbed from the gastrointestinal tract. The time to reach maximum plasma concentration (TCmax) is 1.3 hours. The bioavailability of bilastine when taken orally is 61%. Concomitant food intake reduces the bioavailability of bilastine by 30%. No accumulation of the drug is observed. Connection with blood plasma proteins – 84-90%.
Metabolism and excretion. Bilastine is metabolized slightly; after a single dose, up to 95% of bilastine from the dose taken is excreted unchanged by the kidneys (28.3%) and bile (66.5%). The half-life (T1/2) averages 14.5 hours.
In renal failure of moderate severity (glomerular filtration rate (GFR) 30-50 ml/min/1.73 m2) and severe severity (GFR < 30 ml/min/1.73 m2), the rate of bilastine elimination slows down, which can lead to an increase in the concentration of bilastine in the blood plasma.
Changes in pharmacokinetic parameters do not affect the safety profile of bilastine, since the concentration of bilastine in the blood plasma in patients with renal failure remains within acceptable values.
In case of liver failure, clinically significant changes in the pharmacokinetic parameters of bilastine do not occur, since bilastine is slightly metabolized in the liver.
The pharmacokinetic parameters of bilastine in elderly patients are similar to those in young patients.
Special instructions
Special instructions
In patients with moderate renal failure (GFR 30-50 ml/min/1.73 m2) and severe renal failure (GFR < 30 ml/min/1.73 m2), simultaneous use with P-glycoprotein inhibitors may lead to an increase in the concentration of bilastine in the blood plasma, which increases the risk of side effects.
In this regard, in patients with moderate to severe renal failure, caution must be exercised when using bilastine simultaneously with P-glycoprotein inhibitors (ketoconazole, erythromycin, cyclosporine, ritonavir, diltiazem, etc.).
Impact on the ability to drive vehicles. Wed and fur.:
In a study conducted to evaluate the effect of bilastine at a dose of 20 mg on the ability to drive vehicles, no negative effect of the drug was detected.
However, patients should be warned that in very rare cases, dizziness and drowsiness may occur, which in turn may affect the ability to drive or perform other activities that require increased concentration.
If the described adverse events occur, you should refrain from performing these activities.
Active ingredient
Active ingredient
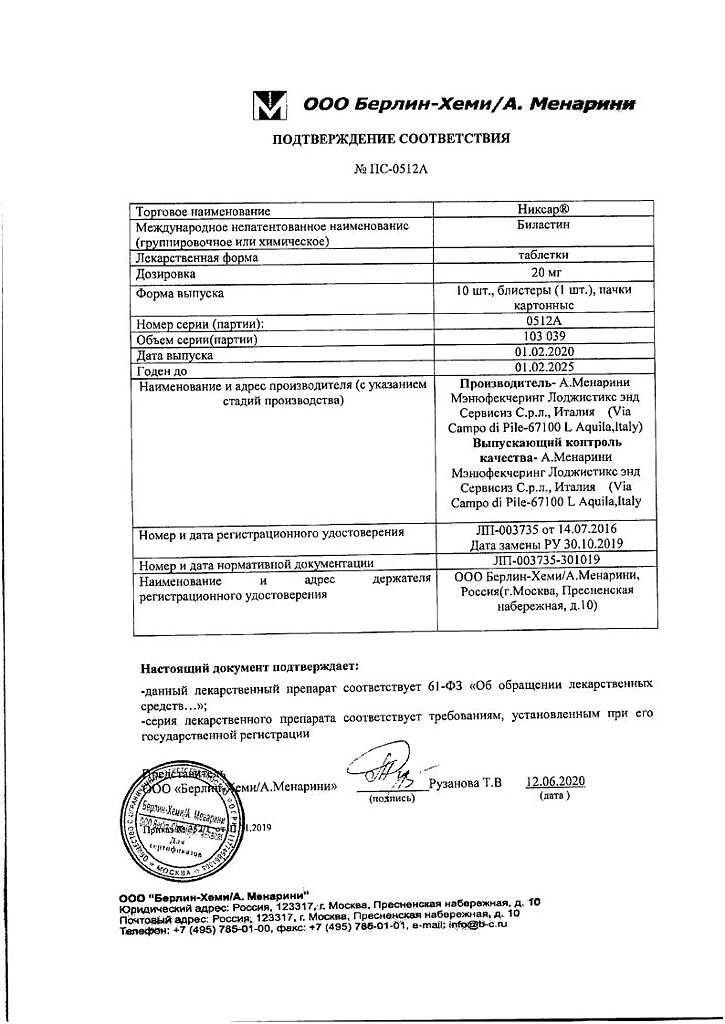
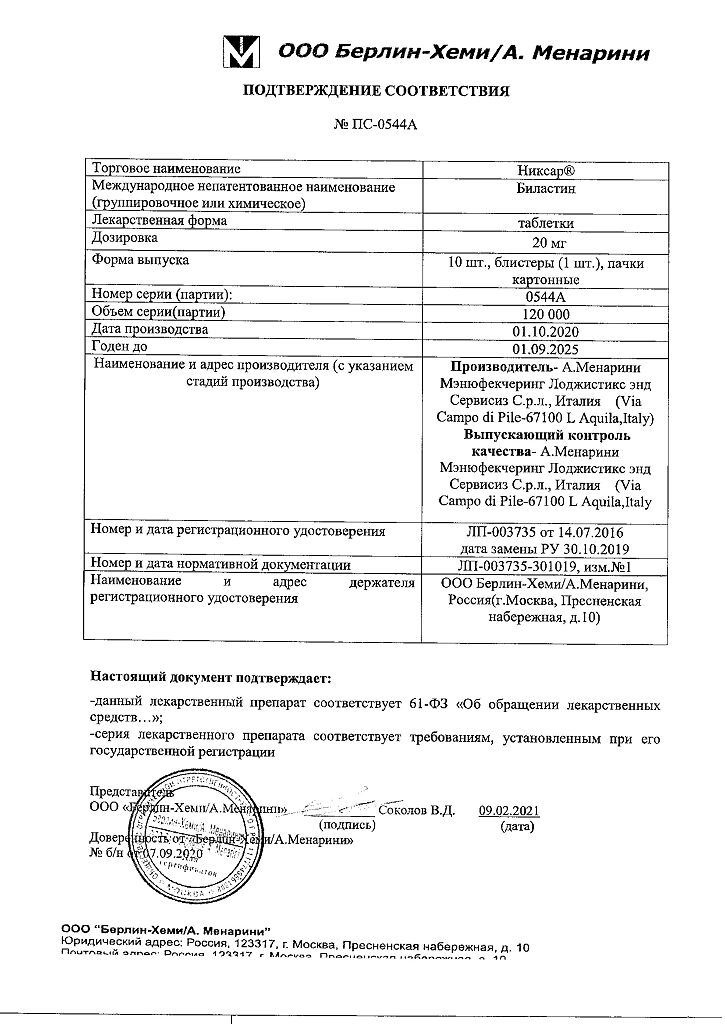
Bilastine
Composition
Composition
Active ingredient:
bilastine – 20.00 mg;
Excipients:
microcrystalline cellulose – 103.00 mg,
sodium carboxymethyl starch (type A) – 1.00 mg,
colloidal silicon dioxide – 0.50 mg,
magnesium stearate – 0.50 mg.
Contraindications
Contraindications
Hypersensitivity to bilastine or auxiliary components of the drug;
age up to 12 years (efficacy and safety have not been established);
pregnancy and breastfeeding period.
Side Effects
Side Effects
Possible side effects are listed below in descending frequency of occurrence: very common (≥ 1/10), common (≥ 1/100, < 1/10), uncommon (≥ 1/1000, < 1/100), rare (≥ 1/10000, < 1/1000), very rare (< 1/10000), including isolated reports.
Gastrointestinal disorders
Uncommon: dry mouth, diarrhea, dyspepsia, gastritis, abdominal pain, upper abdominal pain, stomach discomfort, nausea.
Disorders of the skin and subcutaneous tissues
Uncommon: skin itching.
Nervous system disorders
Often: drowsiness, headache;
Uncommon: dizziness.
Mental disorders
Uncommon: anxiety, insomnia.
Metabolic disorders
Uncommon: increased appetite, weight gain.
Hearing and labyrinth disorders
Uncommon: tinnitus, vertigo.
Disorders of the respiratory system, chest and mediastinal organs
Uncommon: shortness of breath, dry nasal mucosa, discomfort in the nose.
Cardiovascular system disorders
Uncommon: right bundle branch block, sinus arrhythmia, prolongation of the QT interval on the electrocardiogram, other changes on the electrocardiogram.
Infectious and parasitic diseases
Uncommon: herpetic lesions of the oral cavity.
Other:
Uncommon: thirst, increased fatigue, asthenia, fever, increased concentration of triglycerides in the blood plasma, increased concentration of creatinine in the blood plasma, increased activity of liver enzymes (aspartate aminotransferase, alanine aminotransferase, gamma-glutamyltransferase).
Interaction
Interaction
With simultaneous use of bilastine with ketoconazole or erythromycin, the area under the concentration-time curve (AUC) of bilastine increased by 2 times, and the maximum concentration (Cmax) by 2-3 times.
With simultaneous use of bilastine at a dose of 20 mg and diltiazem at a dose of 60 mg, the Cmax of bilastine increased by 50%. Such effects can be explained by interaction at the level of carrier proteins (including P-glycoprotein) responsible for the excretion of drugs from intestinal cells, the substrate of which is bilastine.
With the simultaneous use of bilastine and other drugs that are substrates or inhibitors of P-glycoprotein (for example, cyclosporine), the concentration of bilastine in the blood plasma may increase.
Grapefruit and other fruit juices reduce the bioavailability of bilastine by 30%. This interaction is due to the ability of fruits to suppress the activity of the organic anion transport protein OATP1A2, for which bilastine is a substrate. Medicines that are substrates or inhibitors of OATP1A2 (for example, ritonavir or rifampicin) may reduce the plasma concentration of bilastine.
Bilastine does not enhance the effect of ethanol on the central nervous system.
With the simultaneous use of bilastine and lorazepam, no enhancement or suppressive effect of lorazepam on the central nervous system was detected.
Overdose
Overdose
Symptoms: in clinical studies in healthy volunteers, when using bilastine at a dose 10-11 times higher than the therapeutic dose (220 mg once or 200 mg per day for 7 days), side effects occurred 2 times more often than when using placebo. The most frequently reported symptoms were: dizziness, headache, nausea. Serious side effects, incl. no significant prolongation of the QTc interval was noted. Information obtained from post-marketing studies confirms the safety profile of bilastine described in pre-marketing clinical studies.
Evaluation of the effect of multiple supratherapeutic doses of bilastine (100 mg per day for 4 days) on ventricular repolarization (crossover study of the QT/QTc interval) in 30 healthy volunteers did not reveal a significant prolongation of the QTc segment on the cardiogram.
Treatment: symptomatic and supportive therapy. There is no specific antidote.
Storage conditions
Storage conditions
Store at a temperature not exceeding 30° C.
Keep out of the reach of children!
Manufacturer
Manufacturer
A. Menarini Manufacturing Logistics and Services S, Italy
Additional information
| Conditions of storage | Store at a temperature not exceeding 30° C. Store out of the reach of children! |
|---|---|
| Manufacturer | Faes Pharma S.A., Spain |
| Medication form | pills |
| Brand | Faes Pharma S.A. |
Related products
Buy Nixar, tablets 20 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.