No products in the cart.
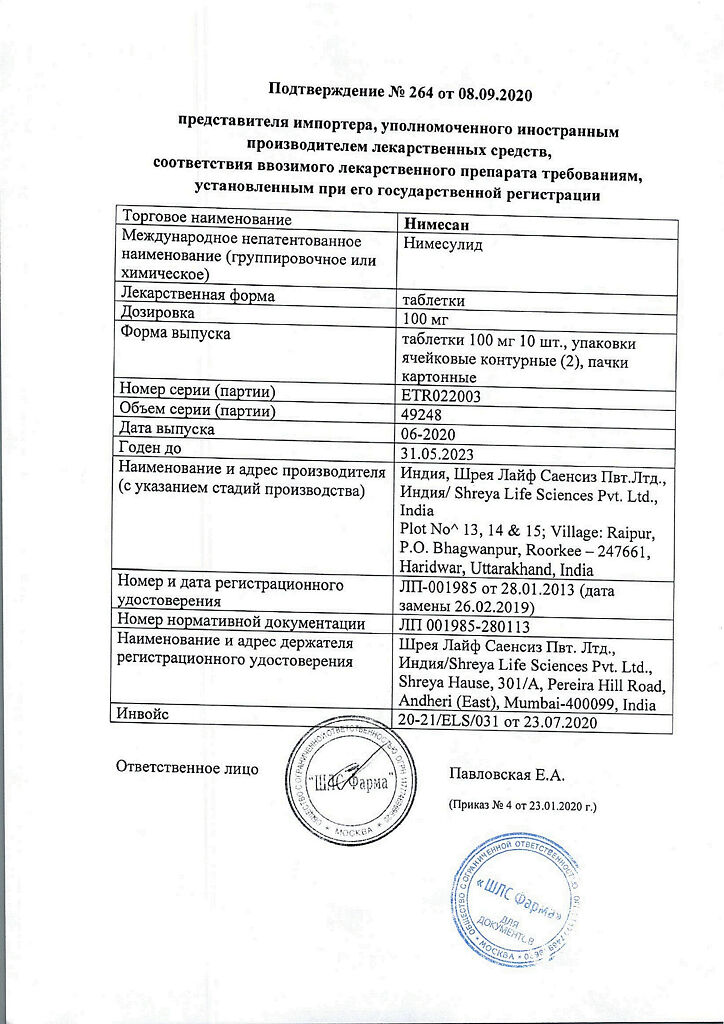
Nimesan, tablets 100 mg 20 pcs
€1.00
Out of stock
(E-mail when Stock is available)
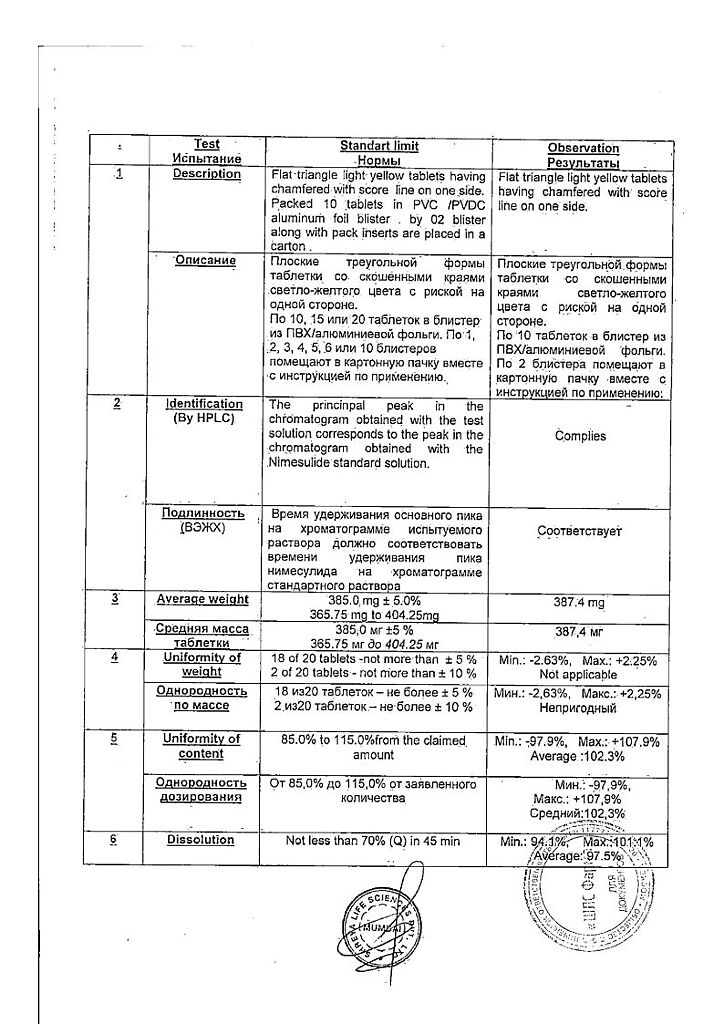
Description
Pharmacotherapeutic group: Non-steroidal anti-inflammatory drug (NSAID)
ATX code: M01AX17
Pharmacological action
Non-steroidal anti-inflammatory drug (NSAID) from the class of sulfonanilides. It is a selective competitive inhibitor of cyclooxygenase-2 (COX-2) – an enzyme involved in the synthesis of prostaglandins – mediators of edema, inflammation and pain. It has anti-inflammatory, analgesic and antipyretic effects.
It reversibly inhibits the formation of prostaglandin E2 both in the focus of inflammation and in the ascending pathways of the nociceptive system, including the spinal cord pain impulse pathways.
Decreases the concentration of short-lived prostaglandin H2, from which prostaglandin E2 is formed under the action of prostaglandin isomerase. Decrease of prostaglandin E2 concentration leads to decrease of activation of prostanoid receptors EP type, which is reflected in analgesic and anti-inflammatory effects.
The drug acts on COX-1 to a small extent, practically not preventing formation of prostaglandin E2 from arachidonic acid in physiological conditions, due to which the number of side effects of the drug is reduced.
The drug also inhibits platelet aggregation by inhibiting the synthesis of endoperoxide and thromboxane A2, inhibits the synthesis of platelet aggregation factor and inhibits plasminogen activation by increasing the concentration of inhibitor-1. Suppresses the release of histamine and also reduces the degree of bronchospasm caused by exposure to histamine and acetaldehyde. It inhibits the release of tumor necrosis factor a, which causes cytokinin formation.
It was shown that nimesulide can inhibit the synthesis of interleukin-6 and urokinase, thus preventing the destruction of cartilage tissue. It inhibits the synthesis of metalloproteases (elastase, collagenase), preventing the destruction of proteoglycans and collagen of cartilage tissue.
It has antioxidant properties, inhibits the formation of toxic products of oxygen decay by reducing the activity of myeloperoxidase. It interacts with glucocorticoid receptors, activating them through phosphorylation, which also increases the anti-inflammatory effect of the drug.
Pharmacokinetics
Absorption when taken orally is high. Food intake reduces the rate of absorption without affecting its degree. Time of reaching maximum concentration of active substance in blood plasma – 1.5-2.5 hours. Binding with plasma proteins is 95%, with red blood cells – 2%, with lipoproteins – 1%, with acidic alpha1-glycoproteins – 1%.
The dose of the drug does not affect the degree of its binding to blood proteins.
The maximum concentration of nimesulide in blood plasma reaches 3.5-6.5 mg/l. The volume of distribution is 0.19-0.35 l/kg. It penetrates into the tissues of the female genitalia, where after a single administration its concentration is about 40% of the plasma concentration. It penetrates well into the acidic environment of the inflammation focus (40%) and synovial fluid (43%).
It easily penetrates through the histo-hematic barriers.
Metabolized in the liver by tissue monooxygenases. The main metabolite, 4-hydroxynimesulide (25%), has similar pharmacological activity.
The half-life of nimesulide is 1.56-4.95 hours, 4-hydroxynimesulide is 2.89-4.78 hours. 4-hydroxynimesulide is excreted by the kidneys (65%) and in the bile (35%).
In patients with renal insufficiency (creatinine clearance 1.8-4.8 l/h or 30-80 ml/min), as well as in children and elderly persons the pharmacokinetic profile of nimesulide does not change significantly.
Indications
Indications
Active ingredient
Active ingredient
How to take, the dosage
How to take, the dosage
Overly, after meals, 50-100 mg twice a day.
In patients with chronic hepatic insufficiency the daily dose is 100 mg.
The lowest effective dose should be used for the shortest possible course.
Children over 12 years of age are prescribed a single dose of 1.5 mg/kg body weight of the child; the maximum daily dose of the drug is 5 mg/kg/day (no more than 200 mg/day).
Interaction
Interaction
The effect of drugs that reduce blood clotting is increased when they are used simultaneously with nimesulide.
Nimesulide may reduce the effect of furosemide. Reduces the therapeutic effect of antihypertensive drugs. Nimesulide may increase the possibility of side effects when concomitant use of methotrexate.
The level of lithium in plasma is increased when concomitant use of lithium and nimesulide. Because of the high degree of binding of nimesulide to plasma proteins, patients treated concomitantly with hydantoin and sulfonamides should be monitored by a physician with monitoring at short intervals.
Nimesulide may increase the effect of cyclosporine on the kidneys.
The use with glucocorticosteroids, serotonin reuptake inhibitors increases the risk of GI bleeding.
Special Instructions
Special Instructions
Contraindications
Contraindications
Side effects
Side effects
The frequency is classified according to the rubric, depending on the occurrence of the case: very often (>10), often (< 10-< 100), infrequently (< 100-< 1000), rarely (< 1000-< 10000), very rarely (< 10000).
Gastrointestinal tract: frequent – diarrhea, nausea, vomiting; infrequent – constipation, flatulence, gastritis; very rare – abdominal pain, stomatitis, tarry stools, gastrointestinal bleeding, ulceration and/or perforation of the stomach or duodenum.
Central nervous system: infrequent – dizziness; rare – feeling of fear, nervousness, nightmares; very rare – headache, somnolence, encephalopathy (Reye syndrome).
Respiratory organs: infrequent – shortness of breath; very rare – bronchial asthma, bronchospasm.
Cardiovascular system: infrequent – arterial hypertension; rare – tachycardia, hemorrhages, “hot flashes”.
Sensory organs: rarely – blurred vision, very rarely – dizziness.
The skin and mucous membranes: infrequent – itching, rash, increased sweating, rare – erythema, dermatitis, very rare – hives, angioedema, swollen face, erythema multiforme, including Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell’s syndrome).
Liver and biliary system: often – increase of “liver” transaminases; very rarely – hepatitis, hepatitis fulminant, jaundice, cholestasis.
Kidney and urinary system: infrequent – edema; rare – dysuria, hematuria, urinary retention, hyperkalemia; very rare – renal failure, oliguria, interstitial nephritis.
Hematopoietic organs: rarely – anemia, eosinophilia; very rarely – thrombocytopenia, pancytopenia, purpura, prolongation of bleeding time.
Allergic reactions: rare – hypersensitivity reactions; very rare – anaphylactoid reactions.
General reactions: rare – general weakness; very rare – hypothermia.
If you experience any other side effects not mentioned above, or if you feel unwell, please talk to your doctor immediately.
Overdose
Overdose
Similarities
Similarities
Additional information
| Shelf life | 3 years. Do not use after the expiration date shown on the package. |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 ° C. Keep out of reach of children. |
| Manufacturer | Shreya Life Sciences Pvt. Ltd, India |
| Medication form | pills |
| Brand | Shreya Life Sciences Pvt. Ltd |
Other forms…
Related products
Buy Nimesan, tablets 100 mg 20 pcs with delivery to USA, UK, Europe and over 120 other countries.