No products in the cart.
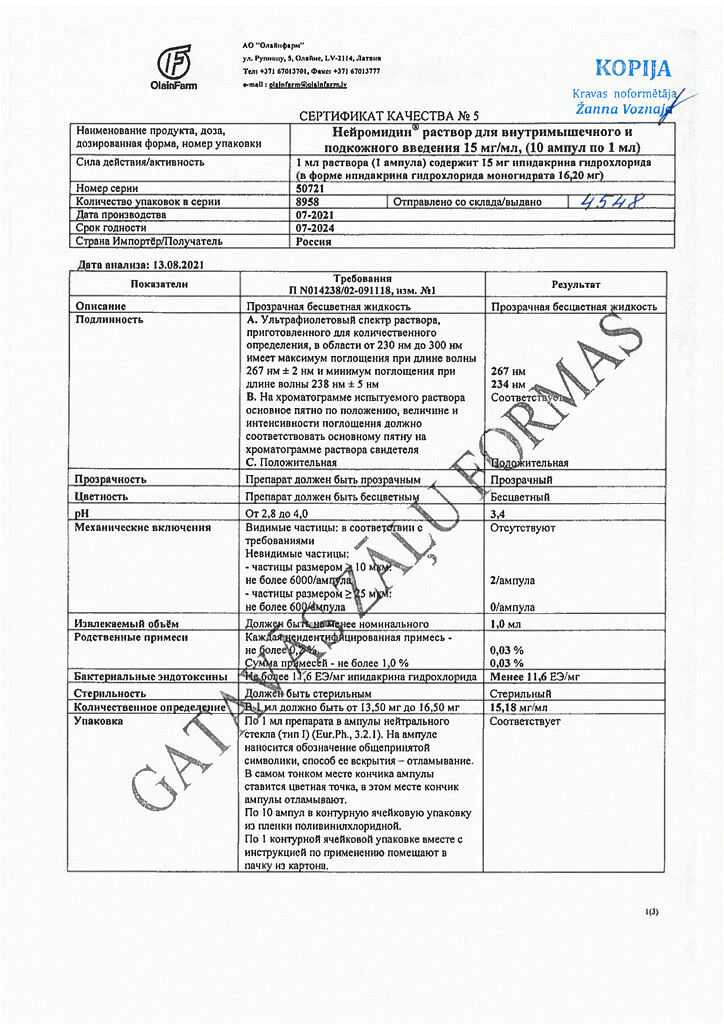
Neuromidine. 15 mg/ml 1 ml 10 pcs
€76.27 €63.56
Description
Neuromidin® has direct stimulating effect on impulse conduction along nerve fibers, inter-neuronal and neuromuscular synapses of CNS and peripheral nervous system. Pharmacological action of Neuromidin® is based on combination of two mechanisms of action: blockade of potassium channels of neuronal and muscle cell membranes; reversible inhibition of cholinesterase in synapses.
Neuromidin® increases the effect on smooth muscles not only of acetylcholine but also of adrenaline, serotonin, histamine and oxytocin.
Neuromidin® has the following pharmacological effects:
– improves and stimulates impulse conduction in the nervous system and neuromuscular transmission;
– enhances smooth muscle organ contractility under the influence of agonists of acetylcholine, adrenaline, serotonin, histamine and oxytocin receptors, except potassium chloride;
– improves memory, inhibits the progressive course of dementia.
In preclinical studies Neuromidin® had no teratogenic, embryotoxic, mutagenic, carcinogenic and immunotoxic effects, nor did it affect the endocrine system.
Pharmacokinetics
After oral, intramuscular and subcutaneous administration, it is rapidly absorbed. Cmax in plasma is reached 1 h after oral administration and 25-30 min after intramuscular or subcutaneous administration.
The binding to plasma proteins is 40-50% of the active substance. It enters the tissues quickly, half-distribution period is 40 minutes.
Metabolized in the liver. It is excreted through the kidneys (mainly by tubular secretion and only 1/3 by glomerular filtration) and extrarenal (through the gastrointestinal tract). T1/2 of Neuromidine® in parenteral administration is 2-3 hours. After parenteral administration 34.8% of the drug dose is excreted unchanged in the urine.
Indications
Indications
Diseases of the peripheral nervous system: mono- and polyneuropathy, polyradiculopathy, myasthenia gravis and myasthenic syndrome of various etiologies. Diseases of the central nervous system: bulbar palsy and paresis; recovery period of organic lesions of the central nervous system, accompanied by motor disorders.
Pharmacological effect
Pharmacological effect
Neuromidin – has a direct stimulating effect on the conduction of impulses along nerve fibers, interneuronal and neuromuscular synapses of the peripheral and central nervous system.
The pharmacological action of Neuromidin is based on a combination of two mechanisms of action:
– blockade of potassium channels in the membrane of neurons and muscle cells;
– reversible inhibition of cholinesterase in synapses.
Neuromidin enhances the effect on smooth muscles of not only acetylcholine, but also adrenaline, serotonin, histamine and oxytocin.
Neuromidin has the following pharmacological effects:
– improves and stimulates impulse conduction in the nervous system and neuromuscular transmission;
– enhances the contractility of smooth muscle organs under the influence of agonists of acetylcholine, adrenaline, serotonin, histamine and oxytocin receptors, with the exception of potassium chloride;
– improves memory, inhibits the progressive course of dementia.
In preclinical studies, Neuromidin did not have teratogenic, embryotoxic, mutagenic, carcinogenic or immunotoxic effects, and did not affect the endocrine system.
Active ingredient
Active ingredient
Ipidacrine
Composition
Composition
1 ml of the drug contains:
active ingredient: ipidacrine hydrochloride monohydrate (in terms of ipidacrine hydrochloride) 15 mg;
excipients: hydrochloric acid, 1M solution (pH stabilizer) to pH 2.8-4.0, water for injection up to 1.0 ml.
Contraindications
Contraindications
Hypersensitivity to any of the components of the drug, epilepsy, extrapyramidal diseases with hyperkinesis, angina pectoris and severe bradycardia, bronchial asthma, mechanical obstruction of the intestine or urinary tract, vestibular disorders, peptic ulcer of the stomach or duodenum in the acute stage, pregnancy (the drug increases the tone of the uterus) and lactation.
Children under 18 years of age (no systematic data on use).
Side Effects
Side Effects
Not known – hypersensitivity reactions (including allergic dermatitis, anaphylactic shock, asthma, toxic epidermal necrolysis, erythema, urticaria, wheezing, laryngeal edema)
Uncommon – dizziness, headache, drowsiness (in case of high doses)
Common: palpitations, bradycardia
Uncommon – increased bronchial secretion
Rarely – diarrhea, pain in the epigastric region
Uncommon – vomiting
Often – drooling, nausea
Often – increased sweating
Uncommon – allergic skin reactions (itching, rash) (when using high doses)
Uncommon – muscle cramps
Uncommon – weakness (when using high doses)
If side effects occur, reduce the dose or interrupt the drug for a short time (1-2 days)
Interaction
Interaction
Neuromidin enhances the sedative effect in combination with drugs that depress the central nervous system.
The action and side effects are enhanced when used together with other cholinesterase inhibitors and M-cholinomimetic drugs.
In patients with myasthenia gravis, the risk of developing a cholinergic crisis increases if Neuromidin is used simultaneously with other cholinergic drugs.
The risk of developing bradycardia increases if β-blockers were used before starting treatment with Neuromidin.
Neuromidin can be used in combination with Cerebrolysin.
Alcohol increases the side effects of the drug.
Overdose
Overdose
Symptoms: decreased appetite, bronchospasm, lacrimation, increased sweating, constriction of the pupils, nystagmus, increased peristalsis of the gastrointestinal tract, spontaneous defecation and urination, vomiting, jaundice, bradycardia, intracardiac conduction disorders, arrhythmias, decreased blood pressure, restlessness, anxiety, agitation, fear, ataxia, convulsions, coma, speech disorders, drowsiness and general weakness.
Treatment: symptomatic therapy is used, m-anticholinergics are used. atropine, cyclodol, metacin, etc.
Storage conditions
Storage conditions
In a place protected from light at a temperature not exceeding 25 ° C. Keep out of the reach of children.
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Sopharma JSC, Bulgaria
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a light-protected place, at a temperature not exceeding 25 °C |
| Manufacturer | Sofarma JSC, Bulgaria |
| Medication form | solution |
| Brand | Sofarma JSC |
Other forms…
Related products
Buy Neuromidine. 15 mg/ml 1 ml 10 pcs with delivery to USA, UK, Europe and over 120 other countries.