No products in the cart.
Naropin, 5 mg/ml 5 pcs.
€1.00
Out of stock
(E-mail when Stock is available)
EAN: 5060249174455
SKU: 211318
Categories: Anesthesia and resuscitation, Anesthesia solutions, Medicine
Description
A long-acting local anesthetic of the amide type. It reversibly blocks voltage-dependent sodium channels and thus prevents generation of impulses in the endings of sensitive nerves and conduction of impulses along the nerve fibers.
The duration of action depends on the route of administration and the dose of the drug.
Pharmacokinetics
Absorption
After administration, ropivacaine is completely absorbed from the epidural space. Absorption is biphasic. Plasma concentration of ropivacaine depends on the dose, route of administration and vascularization of the injection area. Pharmacokinetics of ropivacaine is linear, Cmax is proportional to the administered dose.
Distribution
The pKa of ropivacaine is 8.1; the distribution coefficient is 141 (n-octanol/phosphate buffer pH 7.4).
Vd is 47 l. The average hepatic extraction rate obtained in the experiment is 0.4. Ropivacaine binds in plasma mainly to α1-acid glycoproteins, the unbound fraction is about 6%.
Long-term epidural administration of ropivacaine leads to an increase in total plasma content of ropivacaine, which is due to a postoperative increase in blood levels of α1-acid glycoproteins. However, the concentration of the unbound, pharmacologically active form of ropivacaine in plasma changes to a much lesser extent than the total concentration.
Ropivacaine passes well through the placental barrier. Binding to plasma proteins is lower in the fetus than in the mother.
Metabolism
The drug is actively biotransformed in the body, mainly by hydroxylation. The main metabolite is 3-hydroxy-ropivacaine.
The T1/2 is biphasic and is 14 min (α-phase) and 4 h (β-phase). Total plasma clearance is 440 ml/min. After IV administration about 86% of the dose is excreted with the urine, mainly as metabolites, and only about 1% of the dose is excreted unchanged with the urine. About 37% of 3-hydroxy-ropivacaine is excreted with the urine mainly in the conjugated form.
Indications
Indications
Anesthesia in surgical interventions:
Capitation of acute pain syndrome:
Acute pain management in pediatrics:
Active ingredient
Active ingredient
Composition
Composition
1 ml:
– ropivacaine hydrochloride 2 mg, 7.5 mg, 10 mg
components:
sodium chloride (8.6; 7.5 and 7.1 mg, respectively);
hydrochloric acid or sodium hydroxide (to pH 4-6);
Injectable water.
How to take, the dosage
How to take, the dosage
Interaction
Interaction
Accelerates the toxic effects of other local anesthetics and drugs similar in structure to local anesthetics of the amide type.
Special Instructions
Special Instructions
Anesthesia must be performed by experienced specialists. The availability of equipment and medications for resuscitation measures is mandatory. An intravenous catheter must be in place before large blockages can be performed.
The staff performing the anesthesia must be properly trained and familiar with the diagnosis and treatment of possible side effects, systemic toxic reactions and other possible complications (see “Overdose”).
A complication of unintentional subarachnoid injection may be spinal block with respiratory arrest and decreased BP. Convulsions are more common with brachial plexus block and epidural block, probably due to accidental intravascular injection or rapid absorption at the injection site.
Peripheral nerve blocks may require large volumes of local anesthetic to areas with many vessels and often near major vessels, which increases the risk of intravascular administration and/or rapid systemic absorption, which can lead to high plasma concentrations.
Some procedures involving the use of local anesthetics, such as head and neck injections, may be accompanied by an increased incidence of serious adverse effects, regardless of the type of local anesthetic used. Care must be taken to prevent injection in the area of inflammation.
Patients with intracardiac conduction block II and III degrees, patients with severe renal insufficiency, elderly and frail patients should be cautious when administering the drug.
There have been reports of rare cases of cardiac arrest when using Naropin® for epidural anesthesia or peripheral nerve blocks, especially after accidental intravascular injection, in elderly patients and in patients with concomitant cardiovascular disease.
In some cases, resuscitation measures were difficult. Cardiac arrest generally requires longer resuscitation interventions.
Because Naropin® is metabolized in the liver, caution should be exercised when using the drug in patients with severe liver disease; in some cases, reintroduced doses of the anesthetic may need to be reduced due to delayed elimination.
In patients with renal insufficiency, there is usually no need to adjust the dose when the drug is administered once or when the drug is used for a short period of time. However, acidosis and decreased plasma protein concentrations, which often develop in patients with chronic renal failure, may increase the risk of systemic toxicity of the drug (see section “Dosage and administration”). The risk of systemic toxicity is also increased when using the drug in patients with underweight and patients with hypovolemic shock.
Epidural anesthesia may lead to a decrease in BP and bradycardia. Injection of vasoconstrictors or increased blood pressure may reduce the risk of these side effects. Decreased BP should be promptly corrected by intravenous injection of 5-10 mg of ephedrine, repeated if necessary.
In intra-articular administration of the drug, caution should be exercised if there is suspicion of recent extensive joint trauma or surgery involving the opening of extensive joint surfaces, due to the possibility of increased absorption of the drug and higher plasma concentrations of the drug.
Patients receiving therapy with class III antiarrhythmic drugs (e.g., amiodarone) should be closely monitored and ECG monitoring is recommended due to the risk of increased cardiovascular effects.
The long-term use of Naropin® should be avoided in patients taking strong P4501A2 inhibitors (such as fluvoxamine and enoxacin).
The possibility of cross-sensitivity should be considered if Naropin® is used concomitantly with other amide-type local anesthetics.
Patients on a sodium-restricted diet should take into account the sodium content of the drug.
The use of the drug in newborns requires taking into account the possible immaturity of the organs and physiological functions of newborns. The clearance of the unbound fraction of ropivacaine and pipeloxilidine (PPK) depends on the body weight and age of the child in the first years of life. The effect of age is expressed in the development and maturity of liver function, clearance reaches a maximum value at about 1-3 years of age. The T1/2 of ropivacaine is 5-6 h in newborns and children aged 1 month compared to 3 h in older children. Due to insufficient development of liver functions, systemic exposure of ropivacaine is higher in newborns and moderately higher in children 1 to 6 months of age compared to older children. Significant differences in plasma concentrations of ropivacaine in newborns identified in clinical studies suggest an increased risk of systemic toxicity in this group of patients, especially with prolonged epidural infusion.
The recommended doses for neonates are based on limited clinical data.
When using ropivacaine in neonates, systemic toxicity (monitoring for signs of CNS toxicity, ECG, blood oxygenation control) and local neurotoxicity should be monitored and should be continued after the infusion is completed because of the slow elimination of the drug in neonates.
The use of the drug at concentrations above 5 mg/ml and intrathecal administration of Naropin® in children has not been investigated.
Naropin® has the potential to cause porphyria and should only be used in patients diagnosed with acute porphyria if there is no safer alternative. Appropriate precautions should be taken if patients are hypersensitive.
Chondrolisis has been reported in postoperative prolonged intra-articular infusion of local anesthetics. In most of the cases described, the infusion was given to the shoulder joint. A causal relationship with anesthetic intake has not been established. Naropin® should not be used for prolonged intra-articular infusion.
Impact on the ability to drive vehicles and other mechanisms. In addition to its analgesic effect, Naropin® may have a mild transient effect on motor function and coordination. Considering the side effect profile of the drug, caution must be exercised when driving motor vehicles and performing other potentially dangerous activities requiring increased concentration and rapid psychomotor reactions.
Contraindications
Contraindications
With caution: The drug should be administered to frail elderly patients or patients with severe comorbidities such as intracardiac conduction blockages of degrees II and III (sinoatrial, atrioventricular, intraventricular), advanced liver disease, severe hepatic failure, severe chronic renal failure, in therapy with hypovolemic shock. For these groups of patients regional anesthesia is often preferred. When performing “large” blocks in order to reduce the risk of severe adverse events, it is recommended to optimize the patient’s condition beforehand, as well as to adjust the anesthetic dose.
Caution should be exercised when injecting local anesthetics into the head and neck region because of the possible increased incidence of serious adverse effects.
In intra-articular administration of the drug, caution should be exercised if there is suspicion of recent extensive trauma to the joint or surgery involving the opening of large surfaces of the joint due to the possibility of increased absorption of the drug and higher plasma concentrations of the drug.
Particular attention should be paid when using the drug in children less than 6 months of age due to the immaturity of the organs and functions.
Patients on a sodium-restricted diet need to consider the sodium content of the drug.
Side effects
Side effects
Adverse reactions to Naropin® are similar to those to other amide-type local anesthetics. They should be distinguished from physiological effects resulting from sympathetic nerve blockade during epidural anesthesia, such as decreased BP, bradycardia or effects related to the technique of drug administration, such as local nerve injury, meningitis, post-puncture headache, epidural abscess.
Side effects common to local anesthetics
Central and peripheral nervous system disorders
Neuropathy and spinal cord dysfunction (anterior spinal artery syndrome, arachnoiditis, cauda equina syndrome) are possible, usually related to the technique of regional anesthesia rather than the action of the drug.
The accidental intrathecal administration of an epidural dose can result in complete spinal block.
Serious complications are possible with systemic overdose and unintentional intravascular administration of the drug (see section on Overdose).
Acute systemic toxicity
Naropene can cause acute systemic toxic reactions if high doses are used or if there is a rapid increase in blood concentrations if the drug is accidentally administered intravascularly or in overdose (see section on “Overdose”).
The most frequently reported side effects of the drug
A variety of side effects have been reported, with the vast majority not related to the anesthetic agent used but to the regional anesthetic technique.
The following side effects were the most frequently reported (>1%) and were considered to be of clinical significance regardless of whether a causal relationship with anesthetic use was established decreased BP*, nausea, bradycardia, vomiting, paresthesia, increased body temperature, headache, urinary retention, dizziness, chills, increased BP, tachycardia, hypoesthesia, anxiety.
Very common
Systemic diseases: decreased BP.
Gastrointestinal disorders: nausea.
Often
Nervous system disorders: paresthesia, dizziness, headache.
Cardiovascular system: bradycardia, tachycardia, hypertension.
Gastrointestinal disorders: vomiting.
Hypersensory system disorders: urinary retention.
General: back pain, chills, increased body temperature.
Infrequent
Nervous system disorders: restlessness, symptoms of CNS toxicity (seizures, grand mal seizures, paraesthesia in the perioral area, dysarthria, tongue numbness, visual disturbances, tinnitus, tremor, muscle cramps), hypoesthesia.
Vascular system disorders: fainting.
Respiratory system disorders: shortness of breath, difficulty in breathing.
General: hypothermia.
Rarely
System side: arrhythmia, cardiac arrest.
General: allergic reactions (anaphylactic reactions, angioedema, urticaria).
Overdose
Overdose
Acute systemic toxicity
Inadvertent intravascular administration during plexus blocks or other peripheral blocks has resulted in cases of seizures.
If an intrathecal epidural dose of anesthetic is not administered correctly, a complete spinal block may occur.
The accidental intravascular administration of anesthetic may cause an immediate toxic reaction.
In overdose during regional anesthesia, symptoms of a systemic toxic reaction appear delayed 15-60 minutes after injection due to a slow increase in plasma concentration of the local anesthetic. Systemic toxicity is primarily manifested by CNS and CSF symptoms. These reactions are caused by high blood concentrations of the local anesthetic, which may result from (accidental) intravascular administration, overdose or exceptionally high adsorption from highly vascularized areas. CNS reactions are similar for all amide-type local anesthetics, while cardiovascular reactions are more dependent on the injected drug and its dose.
Central nervous system
The CNS manifestations of systemic toxicity develop gradually: first there are visual disturbances, numbness around the mouth, tongue numbness, hyperacusis, tinnitus, dizziness. Dysarthria, tremor and muscle twitching are more serious manifestations of systemic toxicity and may precede the appearance of generalized seizures (these signs should not be mistaken for the patient’s neurotic behavior). As intoxication progresses, there may be loss of consciousness, seizures lasting from a few seconds to several minutes, accompanied by respiratory distress, rapid development of hypoxia and hypercapnia due to increased muscle activity and inadequate ventilation. In severe cases, respiratory arrest may even occur. Arising acidosis, hyperkalemia, hypocalcemia intensify the toxic effects of the anesthetic.
Thereafter, due to redistribution of the anesthetic from the CNS and its subsequent metabolism and excretion, there is a fairly rapid recovery of function, unless a large dose of the drug was administered.
Cardiovascular system
Disorders on the cardiovascular side are signs of more serious complications. Decreased BP, bradycardia, arrhythmias and, in some cases, even cardiac arrest can occur due to the high systemic concentration of local anesthetics. In rare cases, cardiac arrest is not accompanied by preceding CNS symptoms. In studies on volunteers, intravenous infusion of ropivacaine led to inhibition of conduction and contractility of the heart muscle. CNS symptoms are usually preceded by CNS toxicity that may not be seen if the patient is under sedation (benzodiazepines or barbiturates) or general anesthesia.
In children, early signs of systemic toxicity of local anesthetics may be more difficult to detect due to difficulties in describing symptoms in children or when regional anesthesia is combined with general anesthesia.
Treatment of acute toxicity
In case of the first signs of acute systemic toxicity, the drug should be stopped immediately.
In case of convulsions and CNS depression symptoms, the patient requires adequate treatment to maintain oxygenation, arrest convulsions, maintain cardiac activities. Oxygenation with oxygen should be provided, and if necessary – transfer to AVL. If seizures do not stop after 15-20 seconds, anticonvulsants should be used: sodium thiopental 1-3 mg/kg IV (provides rapid relief of seizures) or diazepam 0.1 mg/kg IV (action is slower compared to that of sodium thiopental). Suxamethonium 1 mg/kg quickly arrests seizures, but intubation and ventilations are required.
In case of depression of cardiac activity (decreased BP, bradycardia), 5-10 mg of ephedrine should be injected intravenously; if necessary, repeat the injection after 2-3 minutes. If circulatory failure or cardiac arrest develops, standard resuscitation measures should be started immediately. It is vital to maintain optimal oxygenation, ventilation and blood circulation, and to correct acidosis. Longer resuscitation interventions may be necessary in cardiac arrest.
In therapy of systemic toxicity in children, doses should be adjusted according to the patient’s age and body weight.
Pregnancy use
Pregnancy use
Pregnancy
There have been no effects of ropivacaine on fertility and reproductive function or teratogenic effects. There have been no studies to evaluate the possible effects of ropivacaine on fetal development in women.
Naropin® can be used in pregnancy only if justified by the clinical situation (in obstetrics, the use of the drug for anesthesia or analgesia is well justified).
Studies on the effects of the drug on reproductive function have been conducted in animals. In studies in rats, ropivacaine had no effect on fertility and reproduction in two generations. When maximum doses of the drug were administered to pregnant rats an increase in offspring mortality was observed in the first three days after delivery, which may be explained by the toxic effect of ropivacaine on the mother, leading to disruption of the maternal instinct.
The teratogenicity studies in rabbits and rats showed no side effects of ropivacaine on organogenesis or early fetal development. Also, in perinatal and postnatal studies in rats receiving the maximum tolerated dose of the drug, there were no adverse effects on late fetal development, labor, lactation, viability, or growth of the offspring. In perinatal and postnatal comparative studies of ropivacaine with bupivacaine, it was shown that, in contrast to ropivacaine, the toxic effects of bupivacaine were observed at significantly lower doses of the drug and at lower blood concentrations of unbound bupivacaine.
Lactation
The excretion of ropivacaine or its metabolites with breast milk has not been studied. Based on experimental data, the dose of the drug administered to the newborn is assumed to be 4% of the dose administered to the mother (drug concentration in milk/plasma concentration of the drug). The total dose of ropivacaine to the infant during breastfeeding is significantly less than the dose that would be delivered to the fetus when the anesthetic is administered to the mother during delivery.
If it is necessary to use the drug during breastfeeding, the balance between the potential benefit to the mother and the possible risk to the infant should be considered.
It is contraindicated in children under 12 years of age.
Additional information
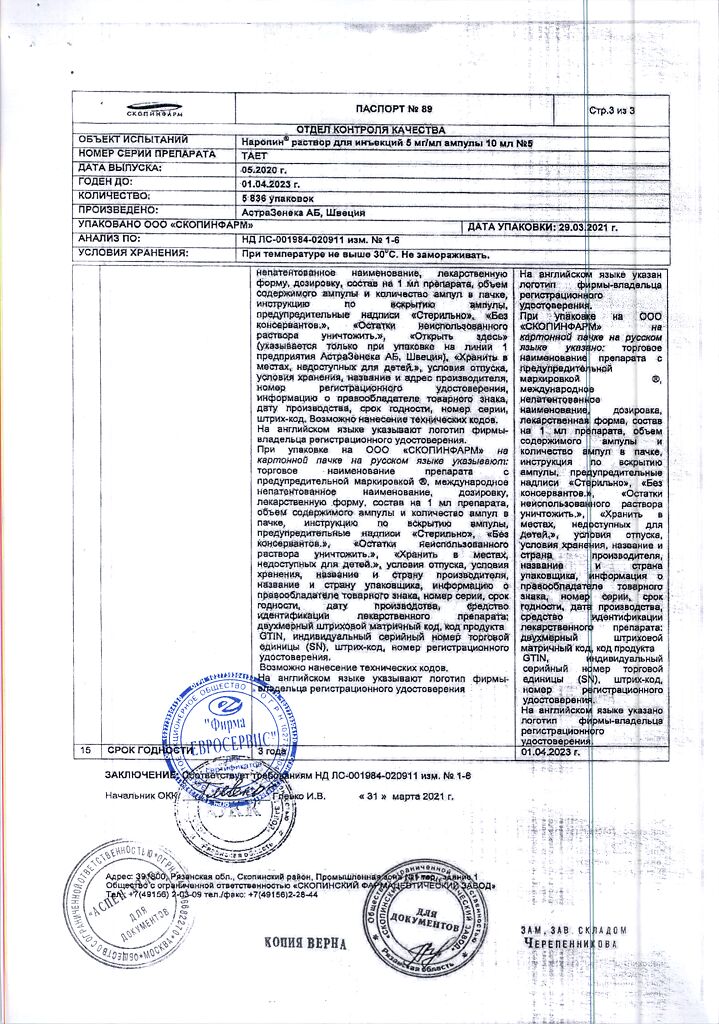
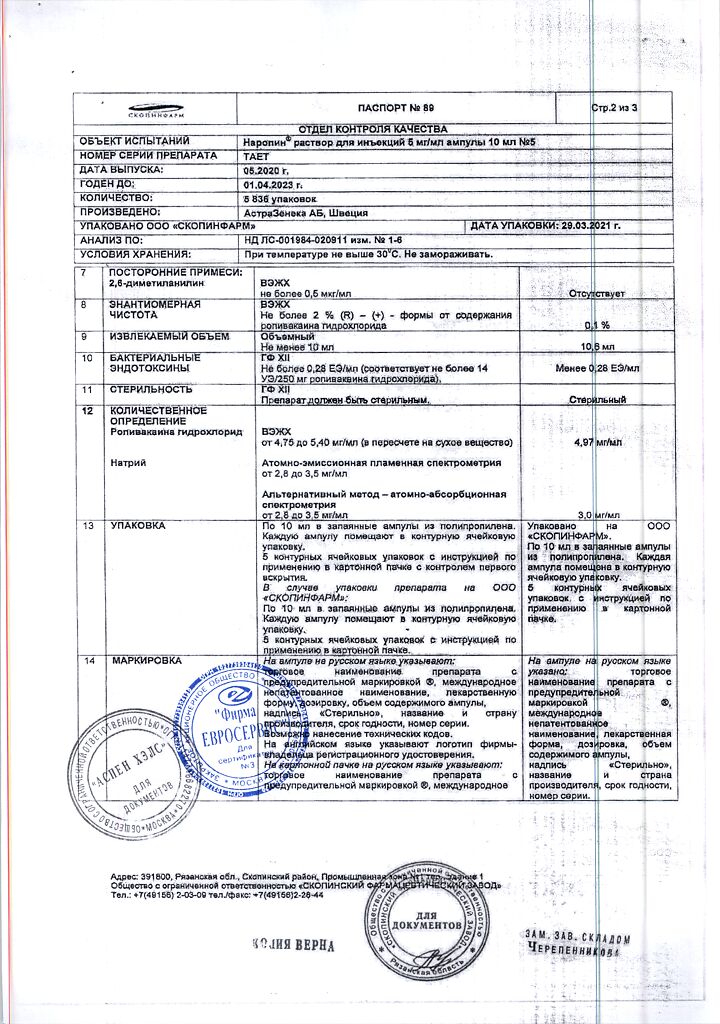
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 30 °C |
| Manufacturer | AstraZeneca AB, Sweden |
| Medication form | solution for injection |
| Brand | AstraZeneca AB |
Related products
Buy Naropin, 5 mg/ml 5 pcs. with delivery to USA, UK, Europe and over 120 other countries.