No products in the cart.
Mydocalm-Richter, 100 mg+2, 5 mg/ml 1 ml 5 pcs
€17.67 €14.73
Description
Midocalm is a myorelaxant.
Pharmacodynamics
Myorelaxant of central action. The mechanism of action is not fully elucidated. It has membrane stabilizing and local anesthetic effect, inhibits conduction of impulses in primary afferent fibers and motor neurons which leads to blocking of spinal mono- and polysynaptic reflexes.
It also probably secondary inhibits mediator release by inhibiting Ca2+ entry into synapses. In the brainstem, it eliminates the facilitation of excitation conduction along the reticulospinal pathway.
It enhances peripheral blood flow independent of CNS influence. The weak antispasmodic and adrenoblocking effect of tolperisone plays a role in the development of this effect.
Pharmacokinetics
Tolperisone is well absorbed from the gastrointestinal tract after oral administration. Cmax is reached after 0.5-1 h, bioavailability is about 20%.
Tolperisone is metabolized in the liver and kidneys. It is excreted in the urine as metabolites (more than 99%). The pharmacological activity of the metabolites is unknown.
Indications
Indications
– Symptomatic treatment of spasticity in adults due to stroke.
– Moderate to severe myofascial pain syndrome (including muscle spasm in dorsopathies).
If there is no improvement or you feel worse after 5 days, you should consult a doctor.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: centrally acting muscle relaxant.
Special instructions
Special instructions
Before using Mydocalm-Richter, consult your doctor.
With caution
The drug should be used with caution in women, patients with hypersensitivity to other drugs or with a history of allergies, and in patients with renal and liver failure.
The most common adverse reactions when using tolperisone in the post-registration period were hypersensitivity reactions. Their severity varies from mild skin reactions to severe systemic reactions, including anaphylactic shock. Symptoms of hypersensitivity reactions may include redness of the skin, rash, hives, itching, swelling of the face, throat, tongue, lips or eyelids, rapid heartbeat, drop in blood pressure or difficulty breathing.
Women and patients with hypersensitivity to other drugs or a history of allergies may be at higher risk of increased sensitivity to tolperisone.
Patients should be alert for any symptoms of hypersensitivity. If symptoms occur, you should immediately stop taking the drug and consult a doctor immediately. Tolperisone should not be re-prescribed after an episode of hypersensitivity to a medicinal product containing tolperisone or lidocaine.
Children and teenagers
Mydocalm-Richter is not intended for use in children and adolescents under 18 years of age.
Special instructions regarding excipients
The drug contains methyl parahydroxybenzoate, which can cause allergic reactions (possibly delayed) and, in exceptional cases, bronchospasm
Impact on the ability to drive vehicles or operate machinery
Tolperisone does not affect the ability to drive vehicles and machines. Patients who experience dizziness, drowsiness, impaired attention, epilepsy, blurred vision, or muscle weakness while using tolperisone should consult a physician.
Active ingredient
Active ingredient
Tolperisone, [Lidocaine]
Composition
Composition
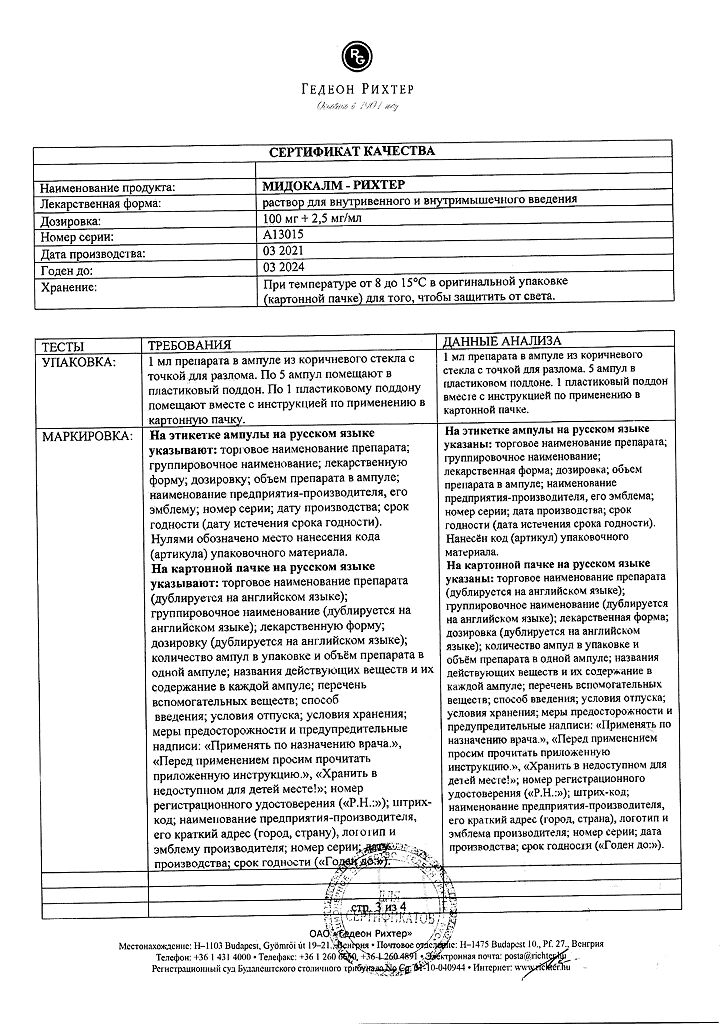
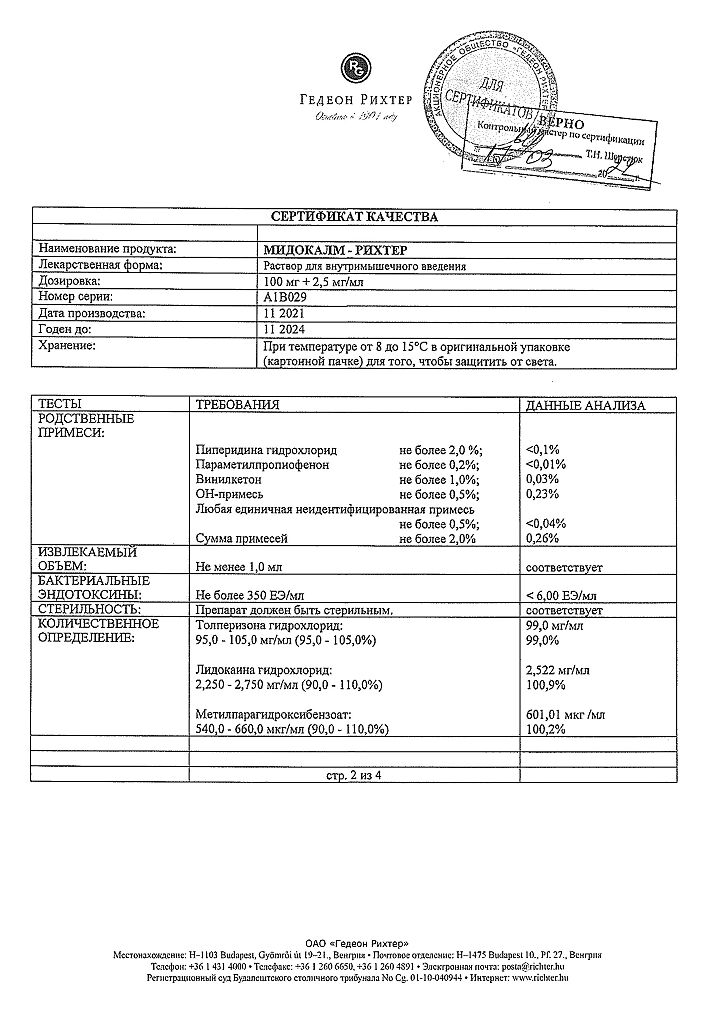
The active ingredients are: tolperisone hydrochloride 100 mg and lidocaine hydrochloride 2.5 mg.
Other excipients are:
methyl parahydroxybenzoate,
diethylene glycol monoethyl ether,
sodium hydroxide 10% solution*,
hydrochloric acid 10% solution*,
water for injections.
*for pH correction if necessary
Pregnancy
Pregnancy
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to become pregnant, consult your doctor before using this drug.
Experimental studies on animals did not reveal the teratogenic effect of tolperisone. Due to the lack of significant clinical data, tolperisone should not be used during pregnancy unless the expected benefit clearly justifies the potential risk to the fetus.
Since there is no data on the excretion of tolperisone in breast milk, its use during breastfeeding is contraindicated.
Contraindications
Contraindications
Do not use Mydocalm-Richter:
– if you are allergic to tolperisone or the chemically similar eperisone or lidocaine or any of the other ingredients of the drug (listed in section 6.1 of the leaflet);
– if you have myasthenia gravis (an immunological disease associated with muscle weakness);
– if you are pregnant or breastfeeding;
– in children and adolescents under 18 years of age.
Side Effects
Side Effects
Description of adverse reactions
Like all medicines, this medicine can cause side effects, although not everyone gets them.
The following reactions have been reported (unknown frequency): angioedema (including facial swelling, swollen lips). Angioedema (an allergic reaction characterized by sudden swelling of the hands, feet, ankles, face, lips, tongue and throat). Trouble swallowing or breathing may also occur.
If serious adverse reactions occur, immediate medical attention may be required. Stop using Mydocalm-Richter and consult your doctor immediately if the following adverse reactions develop:
Rare (occurs in no more than 1 in 1000 patients):
– hypersensitivity reaction, anaphylactic reaction
Very rare (occurs in no more than 1 in 10,000 patients):
– anaphylactic shock
Other possible adverse reactions
Common (occurs in no more than 1 in 10 patients):
– feeling of warmth at the injection site, redness at the injection site
Uncommon (occurs in less than 1 in 100 patients):
– loss of appetite (anorexia)
– insomnia, sleep disorders
– headache, dizziness, drowsiness
– low blood pressure (arterial hypotension)
– abdominal discomfort, diarrhea, dry mouth, dyspepsia, nausea
– muscle weakness, muscle pain (myalgia), pain in the limbs
– movement disorders (dyskinesia)
– weakness, discomfort, fatigue
Rare (occurs in no more than 1 in 1000 patients):
– decreased activity, depression
– impaired attention, tremor, epilepsy, decreased sensitivity (hypesthesia), tingling sensation, numbness (paresthesia), lack of energy, lethargy (lethargy)
– blurred vision
– tinnitus, dizziness (vertigo)
– squeezing pain in the chest (angina), rapid heartbeat (tachycardia), palpitations
– hot flashes
– shortness of breath, nosebleeds, rapid breathing
– pain in the epigastric region, constipation, flatulence, vomiting
– mild liver dysfunction
– allergic dermatitis, increased sweating, itching, urticaria, rash
– discomfort in the limbs
– urinary incontinence (enuresis), protein in urine during laboratory testing (proteinuria)
– feeling of intoxication, feeling of heat, irritability, thirst
– decrease in blood pressure, in laboratory tests: increase in the concentration of bilirubin in the blood, changes in the activity of liver enzymes, decrease in the number of platelets, increase in the number of leukocytes
Very rare (occurs in no more than 1 in 10,000 patients):
– decreased number of red blood cells (anemia), enlarged lymph nodes (lymphadenopathy)
– polydipsia (increased fluid intake)
– confusion
– decreased heart rate (bradycardia)
– decreased bone density (osteopenia)
– chest discomfort
– increase in plasma creatinine concentration
Interaction
Interaction
Tell your doctor if you are taking, have recently taken, or may start taking any other medications.
If you use Mydocalm-Richter simultaneously with the following drugs, they may affect each other, so you should definitely tell your doctor if you are taking any of the following drugs:
– antipsychotics (for example, thioridazine, perphenazine);
– tolterodine (used to treat urinary incontinence);
– antidepressants (for example, venlafaxine, desipramine);
– atomoxetine (used to treat attention deficit hyperactivity disorder (ADHD));
– dextromethorphan (suppresses cough reflex);
– beta blockers (for example, metoprolol, nebivolol);
– niflumic acid and other non-steroidal anti-inflammatory drugs (NSAIDs). Tolperisone enhances the effect of niflumic acid, therefore, with simultaneous use, the possibility of reducing the dose of niflumic acid or other NSAIDs should be considered.
Although tolperisone is a centrally acting drug, its sedative effect is low. In case of simultaneous administration with other centrally acting muscle relaxants, the dose of tolperisone should be reduced.
Overdose
Overdose
The most common symptoms of overdose are drowsiness, gastrointestinal disorders (nausea, vomiting, abdominal pain), tachycardia, hypertension, slowness of movement and dizziness. In severe cases, seizures, difficulty or cessation of breathing, and coma have been reported.
In case of overdose, contact your doctor, pharmacist, or emergency room immediately. There is no specific antidote for tolperisone; symptomatic treatment is recommended.
Storage conditions
Storage conditions
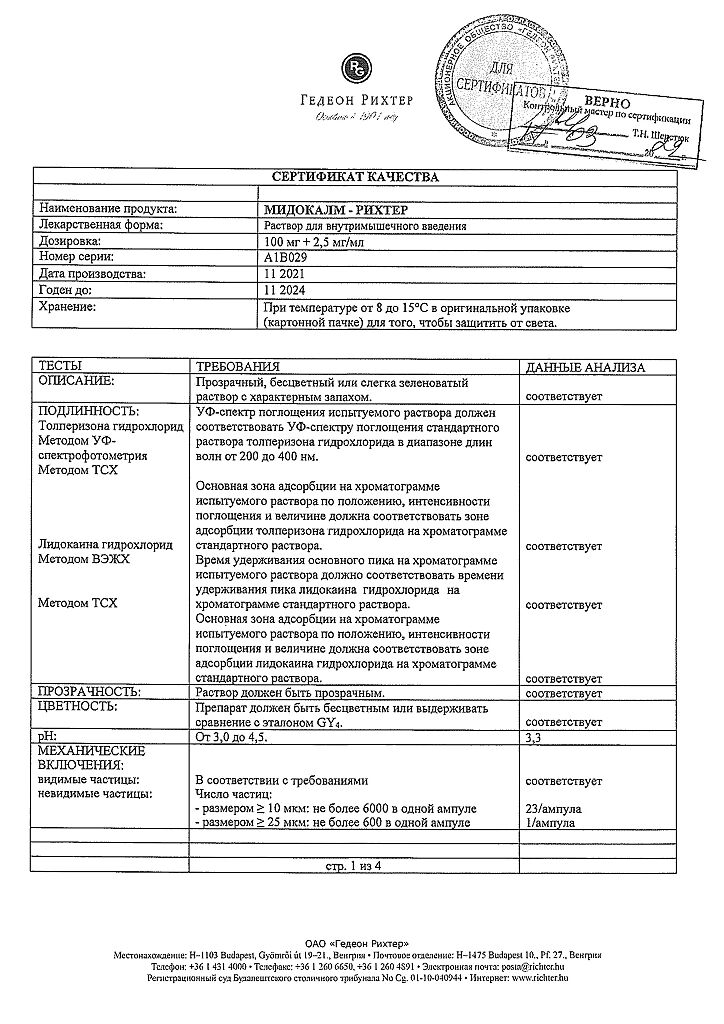
At temperatures from 8 to 15 ºС in the original packaging (cardboard box) in order to protect from light.
Manufacturer
Manufacturer
Gedeon Richter, Hungary
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a light-protected place, at 8-15 °C |
| Manufacturer | Gedeon Richter, Hungary |
| Medication form | solution |
| Brand | Gedeon Richter |
Other forms…
Related products
Buy Mydocalm-Richter, 100 mg+2, 5 mg/ml 1 ml 5 pcs with delivery to USA, UK, Europe and over 120 other countries.