No products in the cart.
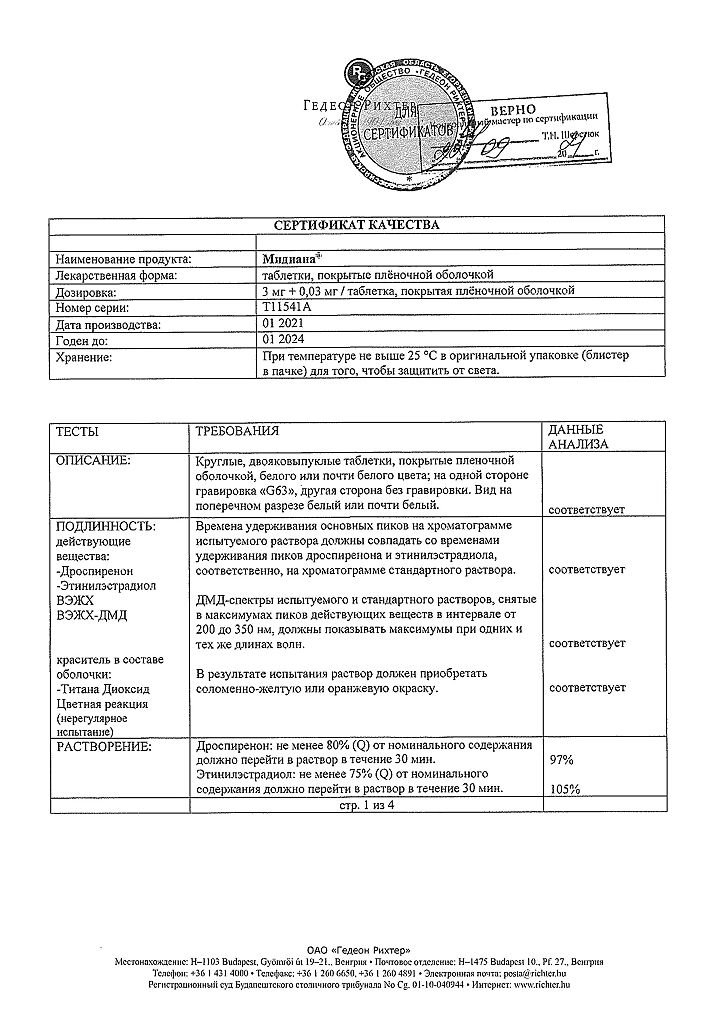
Midiana, 3 mg+0.03 mg 21 pcs
€28.11 €23.42
EAN: 5997001326947
SKU: 213314
Categories: Contraceptive, Gynecology and Obstetrics, Hormonal, Medicine
Description
The contraceptive effect of Midiana® is based on the interaction of different factors, the most important of which are inhibition of ovulation and alteration of the endometrium.
Midiana® is a combination oral contraceptive containing ethinylestradiol and drospirenone. At a therapeutic dose, drospirenone also has antiandrogenic and mild anti-mineralocorticoid properties. It is devoid of any estrogenic, glucocorticoid and antiglucocorticoid activity. This provides drospirenone with a pharmacological profile similar to that of natural progesterone.
There is evidence for a reduced risk of endometrial and ovarian cancer with combined oral contraceptives.
Pharmacokinetics
Drospirenone
Intake. When taken orally, drospirenone is rapidly and almost completely absorbed. Cmax of active substance in serum is 37 ng/ml, Tmax is 1-2 h after a single dose. During 1 cycle of administration, the maximum Css of drospirenone in the serum is about 60 ng/mL and is reached after 7-14 h. Bioavailability ranges from 76 to 85%. Food intake does not affect the bioavailability of drospirenone.
Distribution. After oral administration, there is a biphasic decrease in serum concentration of drospirenone, characterized by T1/2 (1.6±0.7) and (27±7.5) h, respectively. Drospirenone binds to serum albumin and does not bind to sex hormone-binding globulin (hSPH) and corticosteroid-binding globulin (transcortin). Only 3-5% of the total serum concentration of the active ingredient represents free hormone. The ethinylestradiol-induced increase in hGHG does not affect the binding of drospirenone by serum proteins. Mean apparent Vd is (3.7±1.2) L/kg.
Biotransformation. After oral administration, drospirenone undergoes significant metabolism. Most metabolites in plasma are represented by acidic forms of drospirenone, obtained by opening the lactone ring, and 4,5-dihydro-drospirenone-3-sulfate, which are formed without involvement of the cytochrome P450 system. According to in vitro studies, drospirenone is metabolized with negligible involvement of cytochrome P450.
Elimination. The metabolic clearance rate of drospirenone in serum is (1.5±0.2) ml/min/kg. Drospirenone is excreted only in trace amounts unchanged. Drospirenone metabolites are excreted by the kidneys and through the intestine at a ratio of approximately 1.2:1.4. T1/2 for excretion of metabolites by the kidneys and through the intestine is approximately 40 h.
Css. During 1 treatment cycle, the maximum Css (approximately 60 ng/mL) of drospirenone in serum is reached after 7-14 h. There is a 2-3-fold increase in drospirenone concentrations. A further increase in serum concentration of drospirenone is observed after 1-6 cycles of administration, after which no increase in concentration is observed.
Ethinylestradiol
Intake. Ethinylestradiol is rapidly and completely absorbed after oral administration. Cmax after a single dose of 30 mcg is about 100 pg/mL, Tmax is 1-2 h. For ethinylestradiol there is a significant first pass effect with high individual variability. Absolute bioavailability varies and is approximately 45%.
Distribution. The apparent Vd is about 5 l/kg and the binding to plasma proteins is about 98%. Ethinylestradiol induces the synthesis of hGH and transcortin in the liver. Daily administration of 30 mcg of ethinylestradiol increases plasma HGH concentration from 70 to about 350 nmol/L. Ethinylestradiol passes into breast milk in small amounts (about 0.02% of the dose).
Biotransformation. Ethinylestradiol is completely metabolized. The metabolic clearance rate is 5 ml/min/kg.
Elimination. Ethinylestradiol is practically not excreted unchanged. Metabolites of ethinylestradiol are excreted by the kidneys and through the intestine at a ratio of 4:6. The T1/2 for excretion of metabolites is approximately 1 day. The elimination T1/2 is 20 h.
Css. The Css state is reached during the 2nd half of the treatment cycle.
Separate populations
Impact on renal function. The Css of drospirenone in serum in women with mild renal impairment (creatinine Cl 50-80 ml/min) was comparable to that in women with normal renal function (creatinine Cl >80 ml/min). Serum drospirenone concentrations were 37% higher, on average, in women with moderate renal impairment (creatinine Cl 30-50 ml/min) compared to those in women with normal renal function. Drospirenone therapy was well tolerated by women with mild to moderate renal impairment.
The drospirenone treatment had no clinically significant effect on serum potassium concentration.
The effect on liver function. In women with moderate hepatic impairment (Child-Pugh class B), the curve of mean plasma concentration did not correspond to that in women with normal liver function. The Cmax values observed during the absorption and distribution phase were similar. During the end of the distribution phase, the decrease in drospirenone concentrations was approximately 1.8 times greater in volunteers with moderate hepatic impairment compared to those with normal liver function.
After a single dose, total clearance in volunteers with moderate hepatic impairment was approximately 50% lower compared to those with normal liver function.
The observed decrease in drospirenone clearance in volunteers with moderate hepatic impairment does not result in any significant difference in serum potassium concentration. Even with diabetes mellitus and concomitant treatment with spironolactone (two factors that can provoke hyperkalemia in a patient) no increase in serum potassium concentrations above the IOP was observed.
It can be concluded that the drospirenone/ethinylestradiol combination is well tolerated in patients with moderate hepatic impairment (Child-Pugh class B).
Indications
Indications
Preventing pregnancy.
Pharmacological effect
Pharmacological effect
The contraceptive effect of Midiana® is based on the interaction of various factors, the most important of which are inhibition of ovulation and changes in the endometrium.
Midiana® is a combined oral contraceptive containing ethinyl estradiol and drospirenone. At a therapeutic dose, drospirenone also has antiandrogenic and weak antimineralocorticoid properties. It is devoid of any estrogenic, glucocorticoid and antiglucocorticoid activity. This provides drospirenone with a pharmacological profile similar to natural progesterone.
There is evidence of a reduced risk of developing endometrial and ovarian cancer when using combined oral contraceptives.
Pharmacokinetics
Drospirenone
Suction. When taken orally, drospirenone is rapidly and almost completely absorbed. Cmax of the active substance in serum is 37 ng/ml, Tmax is 1–2 hours after a single dose. During 1 cycle of administration, the maximum Css of drospirenone in serum is about 60 ng/ml and is achieved after 7–14 hours. Bioavailability ranges from 76 to 85%. Food intake does not affect the bioavailability of drospirenone.
Distribution. After oral administration, a biphasic decrease in the concentration of drospirenone in the serum is observed, which is characterized by T1/2 (1.6 ± 0.7) and (27 ± 7.5) hours, respectively. Drospirenone binds to serum albumin and does not bind to sex hormone binding globulin (SHBG) and corticosteroid binding globulin (transcortin). Only 3–5% of the total serum concentration of the active substance is free hormone. The increase in SHBG induced by ethinyl estradiol does not affect the binding of drospirenone to serum proteins. The average apparent Vd is (3.7±1.2) l/kg.
Biotransformation. Following oral administration, drospirenone undergoes significant metabolism. Most metabolites in plasma are represented by acid forms of drospirenone, obtained by opening the lactone ring, and 4,5-dihydro-drospirenone-3-sulfate, which are formed without the involvement of the cytochrome P450 system. According to in vitro studies, drospirenone is metabolized with little participation of cytochrome P450.
Elimination. The rate of metabolic clearance of drospirenone in serum is (1.5 ± 0.2) ml/min/kg. Drospirenone is excreted only in trace amounts unchanged. Drospirenone metabolites are excreted by the kidneys and intestines in a ratio of approximately 1.2:1.4. T1/2 for excretion of metabolites by the kidneys and through the intestines is approximately 40 hours.
Css. During 1 cycle of treatment, the maximum Css (approximately 60 ng/ml) of drospirenone in serum is achieved after 7-14 hours. A 2-3-fold increase in the concentration of drospirenone is noted. A further increase in the serum concentration of drospirenone is observed after 1–6 cycles of administration, after which no increase in concentration is observed.
Ethinyl estradiol
Suction. Ethinyl estradiol is rapidly and completely absorbed after oral administration. Cmax after a single dose of 30 mcg is about 100 pg/ml, Tmax is 1–2 hours. Ethinyl estradiol has a significant first-pass effect with high individual variability. Absolute bioavailability varies and is approximately 45%.
Distribution. The apparent Vd is about 5 l/kg, the connection with blood plasma proteins is about 98%. Ethinyl estradiol induces the synthesis of SHBG and transcortin in the liver. With a daily dose of 30 mcg ethinyl estradiol, the plasma concentration of SHBG increases from 70 to approximately 350 nmol/l. Ethinyl estradiol passes into breast milk in small quantities (approximately 0.02% of the dose).
Biotransformation. Ethinyl estradiol is completely metabolized. The rate of metabolic clearance is 5 ml/min/kg.
Elimination. Ethinyl estradiol is practically not excreted unchanged. Metabolites of ethinyl estradiol are excreted by the kidneys and through the intestines in a ratio of 4:6. T1/2 for excretion of metabolites is approximately 1 day. Elimination T1/2 is 20 hours.
Css. The Css state is achieved during the 2nd half of the treatment cycle.
Certain categories of the population
Effect on kidney function. Serum CSS of drospirenone in women with mild renal impairment (creatinine clearance 50–80 ml/min) was comparable to that in women with normal renal function (creatinine clearance >80 ml/min). Serum drospirenone concentrations were on average 37% higher in women with moderate renal impairment (creatinine clearance 30–50 ml/min) compared with those in women with normal renal function. Drospirenone therapy was well tolerated by women with mild to moderate renal impairment.
Drospirenone treatment did not have a clinically significant effect on serum potassium concentrations.
Effect on liver function. In women with moderate hepatic impairment (Child-Pugh class B), the mean plasma concentration curve did not correspond to that in women with normal liver function. The Cmax values observed in the absorption and distribution phases were the same. During the end of the distribution phase, the decrease in drospirenone concentrations was approximately 1.8 times greater in volunteers with moderate hepatic impairment compared with those with normal liver function.
After a single dose, total clearance in volunteers with moderate hepatic impairment was approximately 50% reduced compared to people with normal liver function.
The observed decrease in drospirenone clearance in volunteers with moderate hepatic impairment does not lead to any significant differences in serum potassium concentration. Even with diabetes mellitus and concomitant treatment with spironolactone (two factors that can provoke hyperkalemia in the patient), there was no increase in serum potassium concentration above the ULN.
It can be concluded that the combination of drospirenone/ethinyl estradiol is well tolerated in patients with moderate hepatic impairment (Child-Pugh class B).
Special instructions
Special instructions
If any of the conditions/risk factors listed below currently exist, the potential risks and expected benefits of using a combined oral contraceptive should be carefully weighed on an individual basis and discussed with the woman before she decides to start taking the drug. If any of these conditions or risk factors worsen, intensify, or appear for the first time, a woman should consult her doctor, who may decide whether to discontinue the combined oral contraceptive.
Circulatory system disorders
The incidence of venous thromboembolism (VTE) when using low-dose estrogen combined oral contraceptives (<50 mcg ethinyl estradiol, such as Midiana) is approximately 20 to 40 cases per 100,000 women per year, which is slightly higher than in women not using hormonal contraceptives (5 to 10 cases per 100,000 women), but lower than in women during pregnancy (60 cases per 100,000 pregnancies).
An additional risk of VTE is observed during the first year of combined oral contraceptive use. VTE is fatal in 1-2% of cases.
Epidemiological studies have also found an association between combined oral contraceptive use and an increased risk of arterial thromboembolism. Extremely rare cases of thrombosis of other blood vessels, for example, hepatic, mesenteric, renal, cerebral and retinal vessels, both arteries and veins, have been described in patients taking oral hormonal contraceptives. The cause-and-effect relationship between the occurrence of these side effects and the use of combined oral contraceptives has not been proven.
Symptoms of venous or arterial thrombosis/thromboembolism or cerebrovascular disease may include:
– unusual unilateral pain and/or swelling of the limb;
– sudden severe chest pain, with or without radiation to the left arm;
– sudden shortness of breath;
– sudden coughing attack;
– any unusual, severe, prolonged headache;
– sudden partial or complete loss of vision;
– diplopia;
– slurred speech or aphasia;
– dizziness;
– loss of consciousness with or without a seizure;
– weakness or very significant loss of sensation that suddenly appeared on one half or in one part of the body;
– movement disorders;
– a symptom of “acute abdomen”.
The risk of complications associated with VTE when taking a combined oral contraceptive increases:
– with age;
– in the presence of a family history (venous or arterial thromboembolism in close relatives or parents at a relatively young age); if a hereditary predisposition is suspected, the woman needs to consult a specialist before prescribing a combined oral contraceptive;
– after prolonged immobilization, major surgery, any leg surgery or major trauma. In these situations, it is recommended to stop taking the drug (in the case of planned surgery, at least four weeks before it) and not to resume taking it for two weeks after the end of immobilization. Additionally, antithrombotic therapy may be prescribed if oral hormonal contraceptives have not been discontinued within the recommended time frame;
– for obesity (BMI more than 30 mg/m2).
The risk of arterial thrombosis and thromboembolism increases when taking a combined oral contraceptive:
– with age;
– in smokers (women over 35 years of age are strictly not recommended to smoke if they want to use combined oral contraceptives);
– with dislipoproteinemia;
– for arterial hypertension;
– for migraine;
– for diseases of the heart valves;
– with atrial fibrillation.
The presence of one serious risk factor or multiple risk factors for arterial or venous disease, respectively, may be a contraindication.
Women using combined oral contraceptives should contact their doctor immediately if symptoms of possible thrombosis occur. In cases of suspected or confirmed thrombosis, the combined oral contraceptive should be discontinued. It is necessary to select an adequate method of contraception due to the teratogenicity of anticoagulant therapy (coumarins).
The increased risk of thromboembolism in the postpartum period should be taken into account.
Other diseases that are associated with severe vascular pathology include diabetes mellitus, systemic lupus erythematosus, hemolytic uremic syndrome, chronic inflammatory bowel disease (Crohn’s disease or ulcerative colitis), and sickle cell disease.
An increase in the frequency and severity of migraine during use of combined oral contraceptives (which may precede cerebrovascular events) may be grounds for immediate discontinuation of these drugs.
Tumors
The most significant risk factor for developing cervical cancer is infection with the human papillomavirus. Some epidemiological studies have reported an increased risk of cervical cancer with long-term use of combined oral contraceptives, but there remains controversy regarding the extent to which these findings are attributable to confounding factors such as testing for cervical cancer or use of barrier methods of contraception.
A meta-analysis of 54 epidemiological studies demonstrated that there is a slightly increased relative risk (RR = 1.24) of developing breast cancer diagnosed in women who were using combined oral contraceptives at the time of the study. The excess risk decreases gradually over 10 years after stopping combined oral contraceptives. Because breast cancer is rare in women under 40 years of age, the increase in breast cancer diagnosed in recent years in women taking or taking combined oral contraceptives is small relative to the overall risk of developing breast cancer. These studies do not support a causal relationship between combined oral contraceptives and breast cancer. The observed increased risk may be due to earlier diagnosis of breast cancer in women using combined oral contraceptives, a biological effect of combined oral contraceptives, or a combination of both. Breast tumors in women who had ever taken combined oral contraceptives were clinically less severe than in women who had never taken them.
In rare cases, during the use of combined oral contraceptives, the development of benign liver tumors has been observed, and in even more rare cases, malignant ones. In some cases, these tumors caused life-threatening intra-abdominal bleeding. In the differential diagnosis of a liver tumor, it should be taken into account when a woman taking combined oral contraceptives experiences severe pain in the upper abdomen, liver enlargement, or signs of intra-abdominal bleeding.
Other states
The progesterone component in Midiana is an aldosterone antagonist with the property of retaining potassium. In most cases, there is no increase in potassium concentration. However, in a clinical study in some patients with mild to moderate renal impairment and concomitantly prescribed potassium-retaining medications, serum potassium concentrations were slightly increased when taking drospirenone. Therefore, it is recommended to check the serum potassium concentration in the first cycle of dosing in patients with renal failure and pre-treatment potassium concentration values for ULN, as well as during concomitant use of drugs that retain potassium in the body.
In women with hypertriglyceridemia or a family history of hypertriglyceridemia, an increased risk of pancreatitis cannot be excluded when taking combined oral contraceptives. Although slight increases in blood pressure have been described in many women taking combined oral contraceptives, clinically significant increases have rarely been reported. Only in rare cases is it necessary to immediately stop taking combined oral contraceptives. If, while taking combined oral contraceptives in patients with arterial hypertension, blood pressure values are constantly elevated or do not decrease when taking antihypertensive drugs, the use of combined oral contraceptives should be stopped. If necessary, combined oral contraceptives can be continued if normal blood pressure values are achieved with antihypertensive therapy.
The following conditions develop or worsen both during pregnancy and when taking combined oral contraceptives, but their relationship with taking combined oral contraceptives has not been proven: jaundice and/or itching associated with cholestasis; formation of gallstones; porphyria; systemic lupus erythematosus; hemolytic-uremic syndrome; Sydenham’s chorea; history of herpes during pregnancy; hearing loss associated with otosclerosis.
In women with hereditary angioedema, exogenous estrogens may cause or worsen symptoms of angioedema. For acute or chronic liver dysfunction, it may be necessary to discontinue use of combined oral contraceptives until liver function tests return to normal. Recurrent cholestatic jaundice and/or itching caused by cholestasis, which develops for the first time during pregnancy or previous use of sex hormones, requires discontinuation of combined oral contraceptives.
Although combined oral contraceptives may have an effect on peripheral insulin resistance and glucose tolerance, there is no need to change the therapeutic regimen in diabetic patients using low-dose combined oral contraceptives (containing <50 mcg ethinyl estradiol). However, women with diabetes should be closely monitored by a doctor, especially when starting to take combined oral contraceptives.
An increase in endogenous depression, epilepsy, Crohn’s disease and ulcerative colitis has also been reported with the use of combined oral contraceptives. Chloasma can sometimes develop, especially in women with a history of chloasma during pregnancy. Women prone to chloasma should avoid prolonged exposure to the sun and ultraviolet radiation while taking combined oral contraceptives.
1 tablet contains 48.17 mg of lactose. Patients with hereditary galactose intolerance, lactase deficiency or glucose/galactose malabsorption who are on a lactose-free diet should not take the drug.
Medical examination
Before starting to use hormonal contraceptives, you must consult with your gynecologist and undergo an appropriate medical examination. Further observation and frequency of medical examinations are carried out on an individual basis, but at least once every 6 months.
STDs and HIV infection
Midiana, like other combined oral contraceptives, does not protect against HIV infection and other sexually transmitted diseases.
Reduced efficiency
The effectiveness of combined oral contraceptives may be reduced if pills are missed, gastrointestinal disorders occur, or if other medications are taken at the same time.
Reduced cycle control
While taking combined oral contraceptives, irregular bleeding (spotting or breakthrough uterine bleeding) may occur, especially during the first months of use. Therefore, assessment of any irregular bleeding is only meaningful after an adaptation period of approximately 3 cycles.
If irregular bleeding recurs or develops after previous regular cycles, non-hormonal causes should be considered and adequate diagnostic measures taken to exclude malignancy or pregnancy. These may include diagnostic curettage.
Some women may not develop withdrawal bleeding during a break from combined oral contraceptives. If combined oral contraceptives are taken according to the instructions for taking the drug, then pregnancy is unlikely. However, if combined oral contraceptives have not previously been taken regularly or if there are no consecutive withdrawal bleeds, pregnancy should be ruled out before continuing to take combined oral contraceptives.
Impact on the ability to drive vehicles and operate machinery
There have been no studies examining the effect of the drug on driving ability.
Active ingredient
Active ingredient
Drospirenone, Ethinylestradiol
Composition
Composition
Active ingredients:
ethinylestradiol 30 mcg,
drospirenone 3 mg,
Excipients:
lactose monohydrate,
corn starch,
pregelatinized corn starch,
povidone K25,
magnesium stearate,
Shell:
Opadry II white 85G18490 (polyvinyl alcohol, titanium dioxide, macrogol 3350, talc, soy lecithin).
Pregnancy
Pregnancy
During pregnancy and lactation, the use of Midiana is contraindicated. If pregnancy occurs while on hormonal contraception, immediate discontinuation of the drug is necessary.
The limited available data on unintentional use of combined oral contraceptives suggests no teratogenic effect and an increased risk for children and women during childbirth.
Combined oral contraceptives affect lactation and can reduce the amount and change the composition of breast milk. Small amounts of hormonal contraceptives or their metabolites are found in milk during hormonal contraception and may affect the baby.
The use of combined oral contraceptives is possible after complete cessation of breastfeeding.
Contraindications
Contraindications
Midiana® should not be prescribed if any of the conditions listed below are present. If any of these conditions develop for the first time while taking the drug, its immediate discontinuation is required.
hypersensitivity to the drug or any of its components;
the presence of venous thrombosis currently or in history (deep vein thrombosis, pulmonary embolism);
the presence of arterial thrombosis currently or in history (for example, myocardial infarction);
precursors of thrombosis (including transient ischemic attack, angina), incl. in the anamnesis;
complicated lesions of the heart valve apparatus, atrial fibrillation, uncontrolled arterial hypertension;
major surgery with prolonged immobilization;
smoking over the age of 35;
liver failure;
cerebrovascular diseases currently or in history;
the presence of severe or multiple risk factors for arterial thrombosis (diabetes mellitus with vascular complications, severe arterial hypertension, severe dyslipoproteinemia);
hereditary or acquired predisposition to venous or arterial thrombosis, such as resistance to activated protein C, antithrombin III deficiency, protein C deficiency, protein S deficiency, hyperhomocysteinemia and the presence of antiphospholipid antibodies (anticardiolipin antibodies, lupus anticoagulant);
pancreatitis, incl. in the anamnesis, if severe hypertriglyceridemia was noted;
severe liver disease currently or in history (before normalization of liver tests);
severe chronic renal failure or acute renal failure;
liver tumors (benign or malignant) currently or in history;
hormone-dependent malignant diseases of the reproductive system (genital organs, mammary glands) or suspicion of them;
bleeding from the vagina of unknown origin;
migraine with a history of focal neurological symptoms;
pregnancy or suspicion of it;
lactation period;
hereditary galactose intolerance, lactase deficiency, glucose-galactose malabsorption.
With caution: risk factors for the development of thrombosis and thromboembolism – smoking under the age of 35, obesity, dyslipoproteinemia, controlled arterial hypertension, migraine without focal neurological symptoms, uncomplicated heart valve defects, hereditary predisposition to thrombosis (thrombosis, myocardial infarction or cerebrovascular accident at a young age in one of the immediate relatives); diseases in which peripheral circulatory disorders may occur – diabetes mellitus, systemic lupus erythematosus (SLE), hemolytic-uremic syndrome, Crohn’s disease, ulcerative colitis, sickle cell anemia, phlebitis of the superficial veins; hereditary angioedema; hypertriglyceridemia; liver diseases; diseases that first appeared or worsened during pregnancy or against the background of previous use of sex hormones (including jaundice and/or itching associated with cholestasis, cholelithiasis, otosclerosis with hearing impairment, porphyria, history of herpes during pregnancy, minor chorea – Sydenham disease); chloasma; postpartum period.
Side Effects
Side Effects
When taking combined oral contraceptives, irregular bleeding (spotting or breakthrough bleeding) may occur, especially during the first months of use.
While taking combined oral contraceptives, women experienced other undesirable effects, the connection of which with taking the drugs has not been confirmed, but has not been refuted.
From the digestive system: often – nausea, abdominal pain; infrequently – vomiting, diarrhea.
From the side of the central nervous system: often – asthenic syndrome, headache, decreased mood, mood swings, nervousness; infrequently – migraine, decreased libido; rarely – increased libido.
On the part of the organ of vision: rarely – intolerance to contact lenses (unpleasant sensations when wearing them).
From the reproductive system: often – pain in the mammary glands, engorgement of the mammary glands, menstrual irregularities, vaginal candidiasis, uterine bleeding; infrequently – hypertrophy of the mammary glands; rarely – vaginal discharge, discharge from the mammary glands.
From the skin and its appendages: often – acne; uncommon – rash, urticaria; rarely – erythema nodosum, erythema multiforme.
Other: often – weight gain; infrequently – fluid retention; rarely – weight loss, hypersensitivity reactions.
As with other combined oral contraceptives, in rare cases the development of thrombosis and thromboembolism is possible.
In women with hereditary angioedema, estrogen may cause or worsen symptoms.
Interaction
Interaction
Interactions between oral contraceptives and other drugs may result in breakthrough uterine bleeding and/or decreased contraceptive reliability. The following types of interaction are described in the literature.
Effect on liver metabolism
Some drugs (phenytoin, barbiturates, primidone, carbamazepine and rifampicin) due to the induction of microsomal enzymes can increase the clearance of sex hormones. Possibly the same effect of oxcarbazepine, topiramate, felbamate, ritonavir, griseofulvin and herbal remedy based on St. John’s wort.
The possible effects of HIV protease inhibitors (eg, ritonavir) and non-nucleoside reverse transcriptase inhibitors (eg, nevirapine) and their combinations on hepatic metabolism have been reported.
Effect on enterohepatic recirculation
Clinical observations show that concomitant use with certain antibiotics, such as penicillins and tetracyclines, reduces the enterohepatic recirculation of estrogens, which may lead to a decrease in ethinyl estradiol concentrations.
Women taking any of the above drugs should use a barrier method of contraception in addition to Midiana or switch to any other method of contraception. Women receiving continuous treatment with drugs containing active substances that affect microsomal liver enzymes must additionally use a non-hormonal method of contraception for 28 days after their discontinuation. Women taking antibiotics (other than rifampin or griseofulvin) should temporarily use a barrier method of contraception in addition to the combined oral contraceptive, both while taking the drug and for 7 days after stopping it. If concomitant use of the drug is started at the end of taking a package of Midiana, the next package should be started without the usual break in administration. The main metabolism of drospirenone in human plasma occurs without the involvement of the cytochrome P450 system. Inhibitors of this enzyme system therefore do not affect the metabolism of drospirenone.
The effect of Midiana on other drugs
Oral contraceptives may affect the metabolism of other drugs. In addition, their concentrations in plasma and tissues may change: both increase (for example, cyclosporine) and decrease (for example, lamotrigine).
Based on the results of in vitro inhibition studies and in vivo interaction studies in female volunteers taking omeprazole, simvastatin and midazolam as tracer substrates, an effect of drospirenone 3 mg on the metabolism of other active substances is unlikely.
Other interactions
There is a theoretical possibility of increasing serum potassium concentrations in women receiving oral contraceptives concomitantly with other drugs that increase serum potassium concentrations: ACE inhibitors, angiotensin II receptor antagonists, some NSAIDs (for example, indomethacin), potassium-sparing diuretics and aldosterone antagonists. However, in a study evaluating the interaction of an ACE inhibitor with the combination of drospirenone + ethinyl estradiol in women with moderate hypertension, there was no significant difference between serum potassium concentrations in women receiving enalapril and placebo.
Laboratory research
Taking hormonal contraceptives may affect the results of certain laboratory tests, including biochemical indicators of liver, thyroid, adrenal and kidney function, as well as the concentration of plasma transport proteins, such as corticosteroid binding globulin and lipid/lipoprotein fractions, indicators of carbohydrate metabolism, blood coagulation and fibrinolysis. Changes usually occur within laboratory limits.
Due to its slight antimineralocorticoid activity, drospirenone increases renin activity and plasma aldosterone concentrations.
Overdose
Overdose
There is no information about an overdose of drospirenone and ethinyl estradiol. However, nausea, vomiting and spotting/bleeding from the vagina may occur.
Treatment: there is no specific antidote. Symptomatic treatment should be carried out.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Gedeon Richter, Hungary
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a light-protected place, at a temperature not exceeding 25 °C |
| Manufacturer | Gedeon Richter, Hungary |
| Medication form | pills |
| Brand | Gedeon Richter |
Related products
Gynecology and Obstetrics
Gynecology and Obstetrics
Buy Midiana, 3 mg+0.03 mg 21 pcs with delivery to USA, UK, Europe and over 120 other countries.