No products in the cart.
Metoprolol, tablets 50 mg 50 pcs
€1.68 €1.53
Description
A cardioselective beta1-adrenoblocker without intrinsic sympathomimetic activity. It has hypotensive, antianginal and antiarrhythmic effects.
Limits sinus node automatism, decreases heart rate, slows AV conduction, reduces myocardial contractility and excitability, decreases cardiac minute volume, reduces myocardial oxygen demand. Suppresses the stimulating effect of catecholamines on the heart during physical and psycho-emotional activity.
Causes a hypotensive effect, which stabilizes by the end of the 2nd week of treatment. In tension angina metoprolol reduces the frequency and severity of attacks. Normalizes heart rhythm in supraventricular tachycardia and atrial fibrillation. In myocardial infarction contributes to limiting the area of ischemia of the heart muscle and reduces the risk of fatal arrhythmias, reduces the possibility of recurrence of myocardial infarction.
When used in medium therapeutic doses, it has less pronounced effect on the smooth muscle of the bronchi and peripheral arteries than non-selective beta-adrenoblockers.
Indications
Indications
– Arterial hypertension (as monotherapy or in combination with other antihypertensive agents), including hyperkinetic type, tachycardia;
– Coronary heart disease: myocardial infarction (secondary prevention-complex therapy), prevention of angina attacks;
– Heart rhythm disorders (supraventricular tachycardia, ventricular extrasystole);
– hyperthyroidism (complex therapy);
– prevention of migraine attacks.
Active ingredient
Active ingredient
Composition
Composition
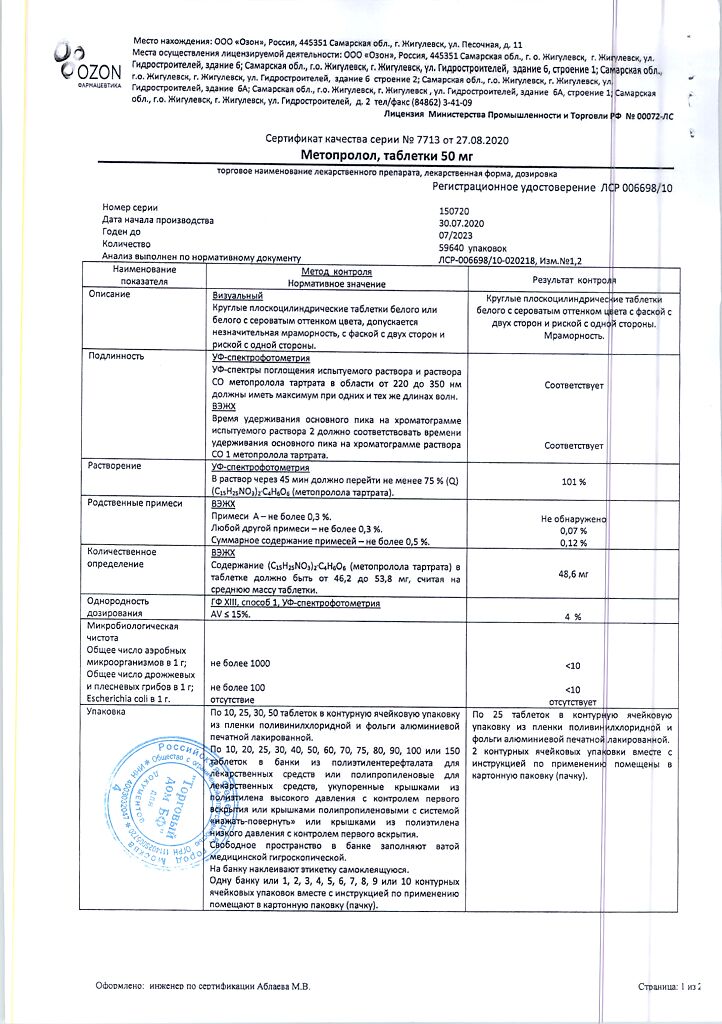
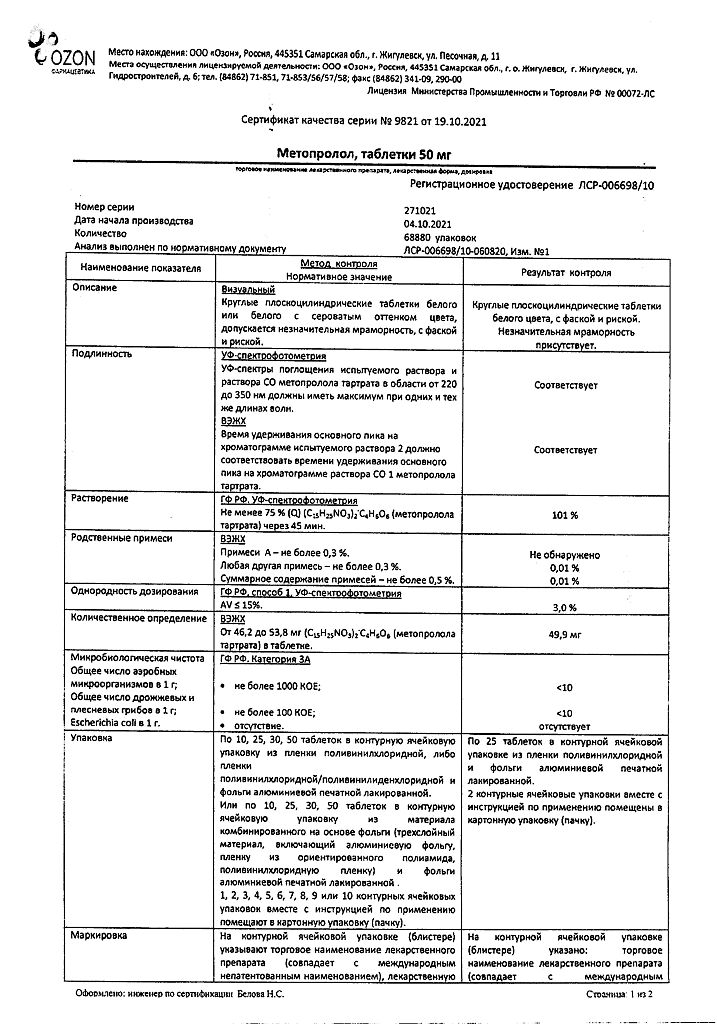
1 tablet metoprolol tartrate 50 mg
Associates:
Silica colloidal anhydrous,
microcrystalline cellulose,
sodium carboxymethyl starch (type A),
magnesium stearate.
Shell composition:
Hypromellose, polysorbate 80, talc, titanium dioxide (E171), crimson dye (Ponceau 4R).
How to take, the dosage
How to take, the dosage
The tablets are taken orally with food or immediately after a meal, without chewing and with fluids.
Arterial hypertension
The initial daily dose is 50-100 mg in 1-2 doses (morning and evening). In case of insufficient therapeutic effect, the daily dose may be gradually increased to 100-200 mg and/or additional prescription of other antihypertensive agents. The maximum daily dose is 200 mg.
Stenocardia, arrhythmias, prevention of migraine attacks – 100-200 mg/day in 2 doses (morning and evening).
Secondary prevention of myocardial infarction – 200 mg/d 2 doses (morning and evening).
Functional disorders of cardiac activity accompanied by tachycardia -100 mg/day 2 doses (morning and evening).
In elderly patients with renal dysfunction and if hemodialysis is necessary, the dose is not changed.
In patients with impaired hepatic function, the dose of the drug should be reduced depending on the clinical condition.
Interaction
Interaction
Combined use with MAO inhibitors is not recommended due to a significant increase in the hypotensive effect. A treatment interval between MAO inhibitors and metoprolol should be at least 14 days.
Concomitant intravenous administration of verapamil may induce cardiac arrest.
The concomitant administration of nifedipine leads to a significant decrease in BP.
Inhalation anesthetics (hydrocarbon derivatives) increase the risk of myocardial dysfunction and arterial hypotension.
Tri- and tetracyclic antidepressants, antipsychotic drugs (neuroleptics), sedatives and hypnotics increase CNS depression.
There is increased CNS depression – with ethanol; summation of the cardiodepressant effect – with anesthetics; increased risk of peripheral circulatory disorders – with ergot alkaloids.
When co-administered with hypoglycemic agents for oral administration, their effect may be reduced; with insulin – increased risk of hypoglycemia, increasing its severity and prolongation, masking some symptoms of hypoglycemia (tachycardia, sweating, increased blood pressure).
When combined with hypotensive agents, diuretics, nitroglycerin or slow calcium channel blockers, a sharp decrease in BP may develop) special caution is required when combined with prazosin); increase in severity of HR depression and suppression of AV conduction – when using metoprolol with verapamil, diltiazem, antiarrhythmic agents (amiodarone), reserpine, alpha-methyl dopa, clonidine, guanfacine, agents for general anesthesia and cardiac glycosides.
If metoprolol and clonidine are taken at the same time, then clonidine should be withdrawn after a few days if metoprolol is withdrawn (due to the risk of withdrawal syndrome).
Hepatic microsomal enzyme inducers (rifampicin, barbiturates) increase metabolism of metoprolol and decrease plasma concentrations of metoprolol and decrease effect.
Inhibitors (cimetidine, oral contraceptives, phenothiazines) increase the plasma concentration of metoprolol.
Allergens used for immunotherapy or allergen extracts for skin testing when combined with metoprolol increases the risk of systemic allergic reactions or anaphylaxis; iodine containing x-ray contrast agents for IV administration increase the risk of anaphylactic reactions.
Decreases clearance of xanthine (except diphylline), especially with initially increased clearance of theophylline due to smoking.
Decreases lidocaine clearance, increases plasma lidocaine concentration.
Magnifies and prolongs the effect of antidepolarizing myorelaxants; prolongs the anticoagulant effect of coumarins.
When co-administered with ethanol, the risk of marked BP decrease increases.
Special Instructions
Special Instructions
Control of patients taking beta-adrenoblockers includes regular monitoring of HR and BP, blood glucose content in diabetic patients. If necessary, for diabetic patients, the dose of insulin or hypoglycemic agents administered orally should be adjusted individually.
The patient should be instructed on how to calculate heart rate and instructed to consult a physician if the heart rate is less than 50 bpm. Dose above 200 mg/day decreases cardioselectivity.
In heart failure, treatment with metoprolol is not started until the compensation stage is reached.
The severity of hypersensitivity reactions may increase (with a history of allergy) and the lack of effect of the usual doses of epinephrine (adrenaline).
It may aggravate the symptoms of peripheral arterial circulatory disorders. The drug should be withdrawn gradually, reducing the dose over 10 days.
The withdrawal syndrome (increased angina and BP) may occur if treatment is stopped abruptly.
Particular attention should be given to patients with angina pectoris when withdrawing the drug. In angina pectoris, the selected dose of the drug should provide an HR at rest within 55-60 bpm, and not more than 110 bpm during exercise.
Patients who wear contact lenses should be aware that treatment with beta-adrenoblockers may decrease tear fluid production.
Methoprolol may mask some clinical manifestations of hyperthyroidism (e.g., tachycardia). Abrupt withdrawal in patients with thyrotoxicosis is contraindicated because it may exacerbate the symptoms.
In diabetes mellitus, it may mask tachycardia caused by hypoglycemia. Unlike non-selective beta-adrenoblockers, it does not practically increase insulin-induced glycemia and does not delay the recovery of blood glucose concentration to normal levels.
In patients with bronchial asthma, beta2-adrenoceptors are used as concomitant therapy; in pheochromocytoma, alpha-adrenoblockers are used.
If it is necessary to perform surgery, the anesthesiologist must be warned about the therapy (choice of general anesthesia agent with minimal negative inotropic effect), cancellation of the drug is not recommended.
Catecholamine-lowering agents (e.g., reserpine) may increase the effects of beta-adrenoblockers, so patients taking these combinations of drugs should be under constant medical supervision for excessive reduction of blood pressure and bradycardia. In elderly patients, regular monitoring of liver function is recommended. Correction of the dosage regimen is required only in elderly patients with increasing bradycardia (less than 50 bpm), marked BP decrease (systolic blood pressure below 100 mmHg), AV-blockade, bronchospasm, ventricular arrhythmias, severe liver function abnormalities, sometimes the treatment must be stopped.
Patients with severe renal insufficiency are advised to monitor renal function.
Patients with depressive disorders taking metoprolol should be monitored particularly; if depression develops as a result of taking beta-adrenoblockers, discontinuing therapy is recommended.
In the absence of sufficient clinical data, the drug is not recommended for use in children.
Impact on the ability to drive and operate machinery
In the beginning of treatment with metoprolol, patients may experience dizziness and fatigue. In this case they should refrain from driving motor transport and engaging in potentially hazardous activities requiring increased concentration and rapid psychomotor reactions. Further determination of the safety of the dose is carried out on an individual basis.
Contraindications
Contraindications
– cardiogenic shock;
– degree II-III AV blockade;
– sinoatrial (SA) blockade;
– sinus node weakness syndrome;
– marked bradycardia;
– decompensated heart failure;
– Prinzmetal angina;
– arterial hypotension (if used for secondary prevention of myocardial infarction – systolic BP less than 100 mm Hg.systolic BP less than 100 mm Hg, HR less than 45 bpm./min);
– concomitant use of MAO inhibitors or concomitant intravenous administration of verapamil;
– lactation;
– age less than 18 years (efficacy and safety have not been established);
– hypersensitivity to metoprolol or other components of the drug, other beta-adrenoblockers.
. With caution – diabetes mellitus, metabolic acidosis, bronchial asthma, chronic obstructive pulmonary disease (pulmonary emphysema, chronic obstructive bronchitis), peripheral occlusive disease (intermittent claudication, Raynaud’s syndrome), chronic hepatic and/or renal failure, myasthenia, pheochromocytoma, AV blockade of 1st degree, thyrotoxicosis, depression (including history).including in anamnesis), psoriasis, pregnancy, old age.
Side effects
Side effects
Side effects depend on the individual sensitivity of the patient. Usually they are minor and disappear after discontinuation of the drug.
Nervous system disorders: increased fatigue, weakness, headache, slowed rate of mental and motor reactions; rarely, paresthesias in the extremities (in patients with intermittent claudication and Raynaud’s syndrome), depression, anxiety, reduced attention span, drowsiness, insomnia, nightmares, confusion or short-term memory disturbance, muscle weakness.
Sensory organs: rarely – decreased vision, decreased tear fluid secretion, dry and sore eyes, conjunctivitis, tinnitus.
Cardiovascular system side: sinus bradycardia, palpitations, decreased BP, orthostatic hypotension, dizziness, sometimes loss of consciousness); rarely – decreased myocardial contractility, temporary worsening of symptoms of chronic heart failure (edema, swelling of the feet and/or lower legs, dyspnea), arrhythmias, angiospasm (increased peripheral circulatory disorders, coldness of the lower extremities, Raynaud’s syndrome), myocardial conduction disorders, cardialgia.
From the digestive system:nausea, vomiting, abdominal pain, dry mouth, diarrhea, constipation, impaired liver function, changes in taste.
Skin side: urticaria, skin itching, rash, exacerbation of psoriasis, psoriasis-like skin reactions, skin hyperemia, exanthema, photodermatosis, increased sweating, reversible alopecia.
In the respiratory system: nasal congestion, difficulty exhaling (bronchospasm when prescribed at high doses – loss of selectivity and/or in predisposed patients), shortness of breath.
From the endocrine system: Hypoglycemia (in patients receiving insulin), rarely: hyperglycemia (in patients with diabetes mellitus), hypothyroidism.
Laboratory parameters: Rarely: thrombocytopenia (unusual bleeding and hemorrhage), agranulocytosis, leukopenia, increased liver enzyme activity; extremely rare: hyperbilirubinemia.
Fetal effects:possible intrauterine growth retardation, hypoglycemia, bradycardia.
Others: back or joint pain, like all beta-adrenoblockers in isolated cases may cause a slight increase in body weight, decreased libido and/or potency.
Overdose
Overdose
Symptoms: severe sinus bradycardia, dizziness, nausea, vomiting, cyanosis, marked BP decrease, arrhythmia, ventricular ecstastole, bronchospasm, syncope, in acute overdose – cardiogenic shock, loss of consciousness, coma, anteoventricular block (up to development of complete transverse block and cardiac arrest), cardialgia.
The first signs of overdose occur 20 minutes to 2 hours after taking the drug.
Treatment: gastric lavage and administration of adsorptive agents; symptomatic therapy: in case of marked BP decrease the patient should be in Trendelenburg position; in case of excessive BP decrease, bradycardia and heart failure – IV, with 2-5 min intervals, beta-adrenostimulants – until the desired effect is achieved or IV 0.5-2 mg of atropine sulfate.
In the absence of a positive effect, dopamine, dobutamine or norepinephrine (noradrenaline).
As a follow-up, prescription of 1-10 mg of glucagon, placement of transvenous intracardiac pacemaker may be indicated. For bronchospasm, IV beta2-adrenoreceptor stimulants should be administered. Metoprolol is poorly excreted by hemodialysis.
Similarities
Similarities
Additional information
| Manufacturer | Ozon, Russia |
|---|---|
| Medication form | pills |
| Brand | Ozon |
Other forms…
Related products
Buy Metoprolol, tablets 50 mg 50 pcs with delivery to USA, UK, Europe and over 120 other countries.