No products in the cart.
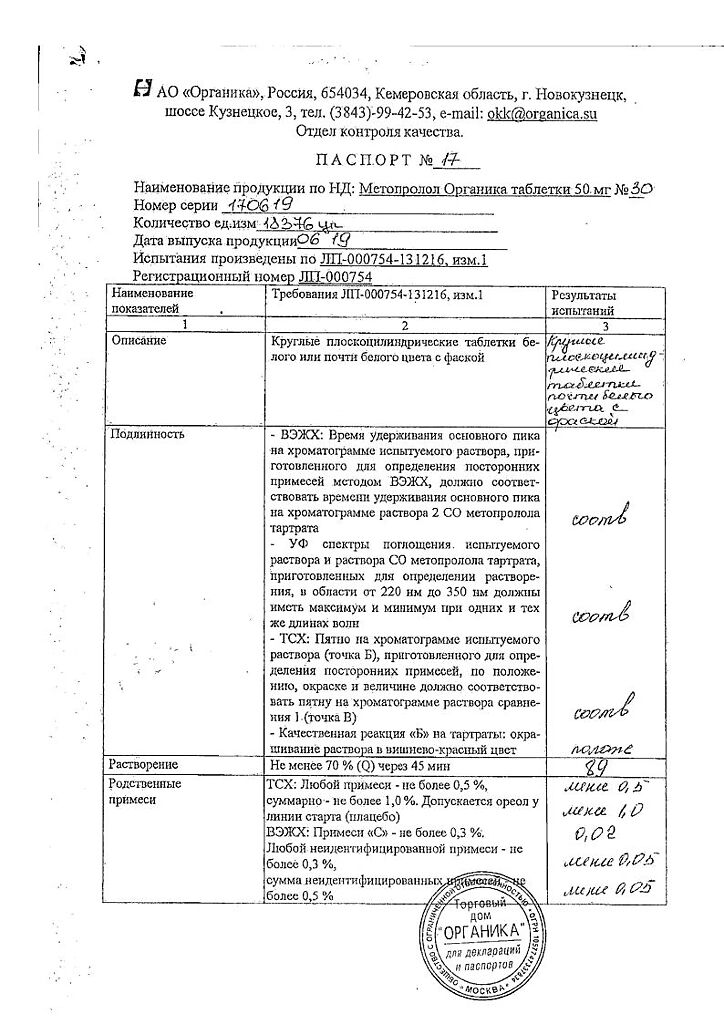
Metoprolol, tablets 50 mg, 30 pcs.
€1.00
Out of stock
(E-mail when Stock is available)
Description
Metoprolol belongs to the cardioselective β-adrenoreceptor blockers with no intrinsic sympathomimetic activity and membrane stabilizing properties. It has hypotensive, antianginal and antiarrhythmic effects.
Blocking in low doses the β-adrenoreceptors of heart, it decreases catecholamine-stimulated formation of cAMP from ATP, decreases intracellular Ca2+ flow, produces negative chrono-, dromo-, batmo- and inotropic action (decreases heart rate, inhibits conduction and excitability and decreases myocardial contractility).
The total peripheral resistance at the beginning of β-adrenoblocker administration (in the first 24 h after oral administration) – increases (as a result of reciprocal increase of α-adrenoreceptor activity and elimination of β-adrenoreceptor stimulation), which returns to baseline after 1-3 days, and decreases with long-term administration.
. Acute antihypertensive effect is due to a decrease in cardiac output, a stable antihypertensive effect develops over 2-3 weeks and is due to a decrease in renin synthesis, and plasma renin accumulation, Inhibition of renin-angiotensin system activity (of great importance in patients with initial renin hypersecretion) and CNS restoration of aortic arch baroreceptors sensitivity (there is no increase of their activity in response to blood pressure decrease) and, finally, reduction of peripheral sympathetic effects. Reduces elevated BP at rest, under physical stress and strain.
The antianginal effect is determined by reducing myocardial oxygen demand as a result of decreased heart rate (prolongation of diastole and improvement of myocardial perfusion) and contractility, as well as decreased myocardial sensitivity to the effects of sympathetic innervation. It reduces the number and severity of angina attacks and increases exercise tolerance. Blood pressure decreases in 15 minutes, maximum in 2 hours and lasts for 6 hours; diastolic BP changes slower: stable decrease is observed after several weeks of regular use.
The antiarrhythmic effect is caused by the elimination of arrhythmogenic factors (tachycardia, increased activity of the sympathetic nervous system, increased content of CAMF, arterial hypertension), the decrease of spontaneous excitation rate of sinus and ectopic pacemakers and AV conduction slowing down (mainly in antegrade and, to smaller extent, in retrograde direction via AV node) and via additional pathways. In supraventricular tachycardia, atrial fibrillation, sinus tachycardia in functional heart disease and hyperthyroidism, it reduces the heart rate, or may even lead to restoration of sinus rhythm. Prevents the development of migraine.
In medium therapeutic doses, unlike non-selective beta-adrenoblockers, it has less pronounced effect on the organs containing β-adrenoreceptors (pancreas, skeletal muscles, smooth muscle of peripheral arteries, bronchi and uterus) and on carbohydrate metabolism. When used in high doses (more than 100 mg/day) it has a blocking effect on both β-adrenoreceptor subtypes.
PHARMACOKINETICS
Metoprolol is rapidly and almost completely (95%) absorbed in the gastrointestinal tract. Cmax in blood plasma is reached 1-2 hours after oral administration. T1/2 is on average 3.5 h (range 1 h to 9 h).
The bioavailability is 50% when administered for the first time and increases to 70% when administered repeatedly. Food intake increases bioavailability by 20-40%. Bioavailability of metoprolol increases in liver cirrhosis. The binding to plasma proteins averages 10%.
The drug penetrates through the blood-brain and placental barriers. It is excreted in breast milk in small amounts.
It is metabolized in the liver. Metabolites have no pharmacological activity. About 5% of the drug is excreted unchanged by the kidneys. Treatment of patients with impaired renal function does not require correction of the drug dose. Impaired liver function slows down the drug metabolism and in cases of poor liver function, the drug dose should be reduced. It is not eliminated by hemodialysis.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
Active ingredients:
metoprolol tartrate;
Associates:
silica colloidal anhydrous,
particular microcrystalline cellulose,
sodium carboxymethyl starch (type A),
magnesium stearate;
Composition of the shell:
Hypromellose, polysorbate 80, talc, titanium dioxide (E171).
How to take, the dosage
How to take, the dosage
Overly, with food or immediately after a meal, without chewing and with liquids.
Arterial hypertension: the initial daily dose is 50-100 mg in 1-2 doses (morning and evening). In case of insufficient therapeutic effect the daily dose may be gradually increased to 100-200 mg and/or other antihypertensive agents may be prescribed in addition.
The maximum daily dose is 200 mg.
Stenocardia, arrhythmias, prevention of migraine attacks: 100-200 mg daily in two doses (morning and evening).
Secondary prevention of myocardial infarction: 200 mg per day in two doses (morning and evening).
Functional cardiac disorders with tachycardia: 100 mg per day in two doses (morning and evening).
In elderly patients, in patients with impaired renal function, and if hemodialysis is necessary, the dose is not changed.
In patients with hepatic impairment the dose of the drug should be reduced depending on the clinical condition.
Interaction
Interaction
Combined use with monoamine oxidase inhibitors (MAOIs) is not recommended due to a significant increase in the hypotensive effect. A treatment interval between MAO inhibitors and metoprolol should be at least 14 days.
Concomitant intravenous administration of verapamil may induce cardiac arrest. Simultaneous administration of nifedipine leads to a significant decrease in BP.
The agents for inhalation anesthesia (hydrocarbon derivatives) increase the risk of myocardial depression and arterial hypotension.
Beta-adrenergic stimulants, theophylline, cocaine, estrogens (sodium retention), indomethacin and other non-steroidal anti-inflammatory drugs (sodium retention and blocking of prostaglandin synthesis by the kidneys) weaken the hypotensive effect.
There is marked an increased suppressive effect on the central nervous system – with ethanol; summation of the cardiodepressant effect – with anesthetics; increased risk of peripheral circulatory disorders – with ergot alkaloids.
When co-administered with oral hypoglycemic agents, their effect may be decreased; with insulin – increased risk of hypoglycemia, its increased severity and prolongation, masking of some symptoms of hypoglycemia (tachycardia, sweating, increased BP).
When combined with hypotensive agents, diuretics, nitroglycerin or slow calcium channel blockers, a sharp decrease in BP may develop (special caution is necessary when combined with prazosin ); increased severity of HR slowing and suppression of atrioventricular conduction when using metoprolol with verapamil, diltiazem, antiarrhythmic agents (amiodarone), reserpine, clonidine, guanfacine, agents for general anesthesia and cardiac glycosides.
Hepatic microsomal enzyme inducers (rifampicin, barbiturates) lead to increased metabolism of metoprolol and decreased plasma concentrations of metoprolol and reduced effect.
Inhibitors (cimetidine, oral contraceptives, phenothiazines) – increase plasma concentrations.
Allergens used for immunotherapy or allergen extracts for skin tests when combined with metoprolol increase the risk of systemic allergic reactions or anaphylaxis; iodine containing x-ray contrast agents for IV administration increase the risk of anaphylactic reactions.
Decreases clearance of xanthine (except diphylline), especially in patients with initially increased clearance of theophylline due to smoking. Reduces lidocaine clearance, increases plasma lidocaine concentrations. Enhances and prolongs the effect of antidepolarizing myorelaxants; prolongs the anticoagulant effect of coumarins.
In co-administration with ethanol the risk of significant BP decrease increases.
Contraindications
Contraindications
Side effects
Side effects
Nervous system disorders: increased fatigue, weakness, headache, slowed rate of mental and motor reactions; rarely – paresthesias in the extremities (in patients with claudication and Raynaud’s syndrome), depression, anxiety, reduced attention, drowsiness, insomnia, nightmares, confusion or short-term memory loss, muscle weakness.
Sensory organs: rarely – decreased vision, decreased tear fluid secretion, dry and sore eyes, conjunctivitis, tinnitus.
Cardiovascular system disorders: sinus bradycardia, palpitations, decreased BP, orthostatic hypotension, dizziness, sometimes loss of consciousness); rarely – decreased myocardial contractility, temporary worsening of symptoms of chronic heart failure (edema, swelling of the feet and/or lower legs, dyspnea), arrhythmias, angiospasm (increased peripheral circulatory disorders, coldness of the lower extremities, Raynaud’s syndrome), myocardial conduction disorders, cardialgia.
Digestive system disorders: nausea, vomiting, abdominal pain, dry mouth, diarrhea, constipation, liver function disorders, change in taste.
Skin disorders: urticaria, skin itching, rash, exacerbation of psoriasis, psoriasis-like skin reactions, skin hyperemia, exanthema, photodermatosis, increased sweating, reversible alopecia.
Respiratory system: nasal congestion, difficulty in exhaling (bronchospasm when prescribed in high doses – loss of selectivity and/or in predisposed patients), dyspnea.
Endocrine system: hypoglycemia (in patients receiving insulin), rarely: hyperglycemia (in patients with diabetes), hypothyroidism.
Laboratory parameters: rare: thrombocytopenia (unusual bleeding and hemorrhage), agranulocytosis, leukopenia, increased activity of liver enzymes; extremely rare: hyperbilirubinemia.
Fetal effects: possible intrauterine growth retardation, hypoglycemia, bradycardia.
Others: back or joint pain, as all beta-adrenoblockers in single cases may cause a slight increase in body weight, decreased libido and/or potency.
Overdose
Overdose
Symptoms: severe sinus bradycardia, dizziness, nausea, vomiting, cyanosis, marked BP decrease, arrhythmia, ventricular extrasystole, bronchospasm, syncope, in acute overdose – cardiogenic shock, loss of consciousness, coma, atrioventricular block (up to development of complete transverse block and cardiac arrest), cardialgia. The first signs of overdose occur 20 minutes to 2 hours after taking the drug.
Treatment: gastric lavage and administration of adsorptive agents; symptomatic therapy: in case of marked BP decrease the patient should be in Trendelenburg position; in case of excessive BP decrease, bradycardia and heart failure – intravenous, with 2-5 min intervals, ba-adrenostimulants – until the desired effect is achieved or intravenous 0.5-2 mg of atropine sulfate. If there is no positive effect, dopamine, dobutamine or norepinephrine (noradrenaline). As a follow-up, prescription of 1-10 mg of glucagon, placement of transvenous intracardiac pacemaker is possible. In bronchospasm, IV beta 1 -adrenoreceptor stimulants should be administered. Slow IV administration of diazepam in case of seizures. Hemodialysis is not effective.
Similarities
Similarities
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a light-protected place, at a temperature not exceeding 25 °C |
| Manufacturer | Organika, Russia |
| Medication form | pills |
| Brand | Organika |
Other forms…
Related products
Buy Metoprolol, tablets 50 mg, 30 pcs. with delivery to USA, UK, Europe and over 120 other countries.