No products in the cart.
Description
Acute lymph leukemia, trophoblastic disease, skin cancer, cervical and vulvar cancer, esophageal cancer, squamous cell head and neck cancer, renal pelvis and urothelial cancer, osteogenic and soft cell sarcoma, Ewing’s sarcoma, lung cancer, breast cancer, testicular and ovarian germ cell tumors, liver cancer, kidney cancer, retinoblastoma, medulloblastoma, penile cancer, and lymphogranulematosis.
Severe forms of psoriasis (in case of ineffectiveness of standard therapy).
The severe form of rheumatoid arthritis (in case of ineffectiveness of standard therapy).
Indications
Indications
Acute lymphocytic leukemia, trophoblastic disease, skin cancer, cervical and vulvar cancer, esophageal cancer, squamous cell cancer of the head and neck, renal pelvis and ureter cancer, osteogenic and soft cell sarcoma, Ewing’s sarcoma, lung cancer, breast cancer, testicular and ovarian germ cell tumors, liver cancer, kidney cancer, retinoblastoma, medulloblastoma, penile cancer, lymphogranulomatosis.
Severe forms of psoriasis (in case of ineffectiveness of standard therapy).
Severe form of rheumatoid arthritis (in case of ineffectiveness of standard therapy).
Pharmacological effect
Pharmacological effect
Antitumor drug.
Special instructions
Special instructions
Use for liver dysfunction
Contraindicated in cases of severe liver dysfunction.
Use for renal impairment
Contraindicated in cases of severe renal impairment.
Methotrexate should not be used for ascites, pleural effusion, gastric and duodenal ulcers, ulcerative colitis, gout or nephropathy (including a history).
It is not recommended for use in patients with chickenpox (including recent or after contact with sick people), herpes zoster and other acute infectious diseases.
Before starting therapy and during treatment, the peripheral blood picture, liver and kidney function, and chest x-ray should be monitored.
When treating rheumatoid arthritis or psoriasis, a detailed general blood test should be done at least once a month, and laboratory tests of liver or kidney function at least once every 1-2 months.
When used for psoriasis, local treatment of the disease should not be interrupted. In case of overdose, it is recommended to use calcium folinate (but no later than after 4 hours).
When conducting combination antitumor therapy, special caution should be exercised when using methotrexate in high doses simultaneously with drugs that have a nephrotoxic effect (for example, cisplatin).
It is not recommended to vaccinate patients and their families.
Caution should be used when combining methotrexate (even in low doses) with acetylsalicylic acid.
Experimental studies have established the carcinogenic and mutagenic effects of methotrexate.
Active ingredient
Active ingredient
Methotrexate
Composition
Composition
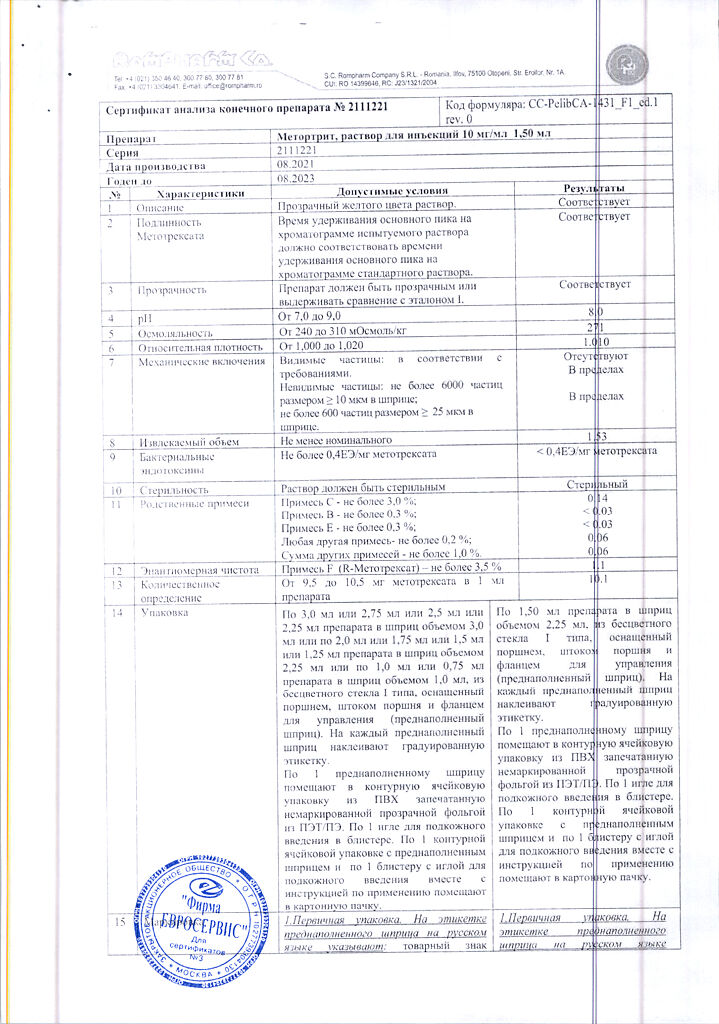
Solution for injection is yellow, transparent.
1 ml: methotrexate disodium 10.97 mg, which corresponds to the content of methotrexate 10 mg
Excipients:
sodium chloride – 7 mg,
sodium hydroxide solution 2M – 1.76 mg (22 µl),
sodium benzoate solution 1M – up to pH – 8.5±0.1,
water for d/i – up to 1 ml.
Contraindications
Contraindications
Severe liver and/or kidney dysfunction, leukopenia, thrombocytopenia, pregnancy. Methotrexate should not be used in immunocompromised conditions.
Side Effects
Side Effects
From the digestive system: possible ulcerative stomatitis, anorexia, gingivitis, pharyngitis, nausea; rarely – diarrhea, melena, enteritis, pancreatitis; in some cases (with long-term daily use) – liver necrosis, cirrhosis, fatty atrophy, periportal liver fibrosis.
From the hematopoietic system: leukopenia, anemia, thrombocytopenia.
From the side of the central nervous system: feeling of fatigue, dizziness; rarely – headache, aphasia, drowsiness, convulsions.
From the reproductive system: disorders of oogenesis and spermatogenesis, oligospermia, menstrual irregularities, decreased libido, impotence.
From the urinary system: hematuria, cystitis, severe renal dysfunction.
Allergic reactions: chills, decreased resistance to infection; rarely – urticaria, toxic epidermal necrolysis, Stevens-Johnson syndrome.
Dermatological reactions: skin rash, photosensitivity, pigmentation disorders, telangiectasia, acne, furunculosis.
Interaction
Interaction
When used simultaneously with vitamin preparations containing folic acid or its derivatives, the effectiveness of methotrexate may be reduced.
The simultaneous use of NSAIDs in high doses can lead to an increase in the concentration of methotrexate in plasma and to a prolongation of its elimination period, as well as an increase in the concentration of methotrexate not bound to plasma albumin, which in turn increases the toxic effects of methotrexate (primarily on the gastrointestinal tract and hematopoietic system).
When used simultaneously with penicillins, methotrexate (even in low doses) may increase its toxic effects.
When used simultaneously with sulfonamides, especially with co-trimoxazole, there is a risk of increased myelosuppressive effects.
When using nitrous oxide in patients receiving methotrexate, severe unpredictable myelodepression and stomatitis may develop.
When used simultaneously with methotrexate, valproic acid may reduce its concentration in the blood plasma.
Cholestyramine binds methotrexate, reduces its enterohepatic recirculation, which leads to a decrease in its concentration in the blood plasma.
When used simultaneously with mercaptopurine, it is possible to increase its bioavailability due to impaired metabolism during the “first pass” through the liver.
Neomycin and paromomycin reduce the absorption of methotrexate from the gastrointestinal tract.
In patients receiving omeprazole, the concentration of methotrexate in the blood plasma may increase.
When used simultaneously with probenecid, a 3-4 fold increase in the concentration of methotrexate in the blood plasma is possible due to a decrease in its renal excretion.
With simultaneous use of methotrexate with retinoids, the risk of hepatotoxicity may increase.
Salicylates potentiate the effect of methotrexate by reducing its renal excretion.
After a course of treatment with tetracycline, methotrexate, used even in low doses, can have a toxic effect.
With sequential administration of methotrexate and fluorouracil, synergistic action is possible; fluorouracil administered before methotrexate may reduce its toxicity.
Cisplatin is nephrotoxic and may therefore reduce the renal excretion of methotrexate, resulting in increased toxicity.
Increased toxicity is possible when using cyclosporine in patients receiving methotrexate.
Manufacturer
Manufacturer
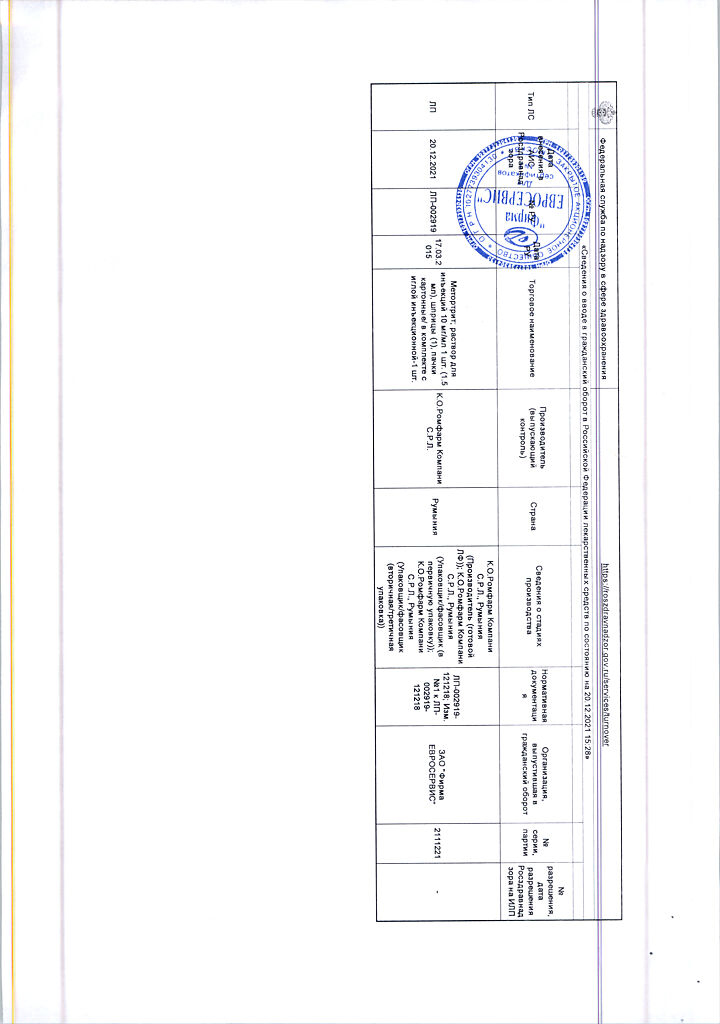
K.O.Rompharm Company S.R.L., Romania
Additional information
| Manufacturer | C.O.Rompharm Company S.R.L., Romania |
|---|---|
| Medication form | solution for injection |
| Brand | C.O.Rompharm Company S.R.L. |
Other forms…
Related products
Buy Methotrite, 10 mg/ml 1.5 ml with delivery to USA, UK, Europe and over 120 other countries.