No products in the cart.
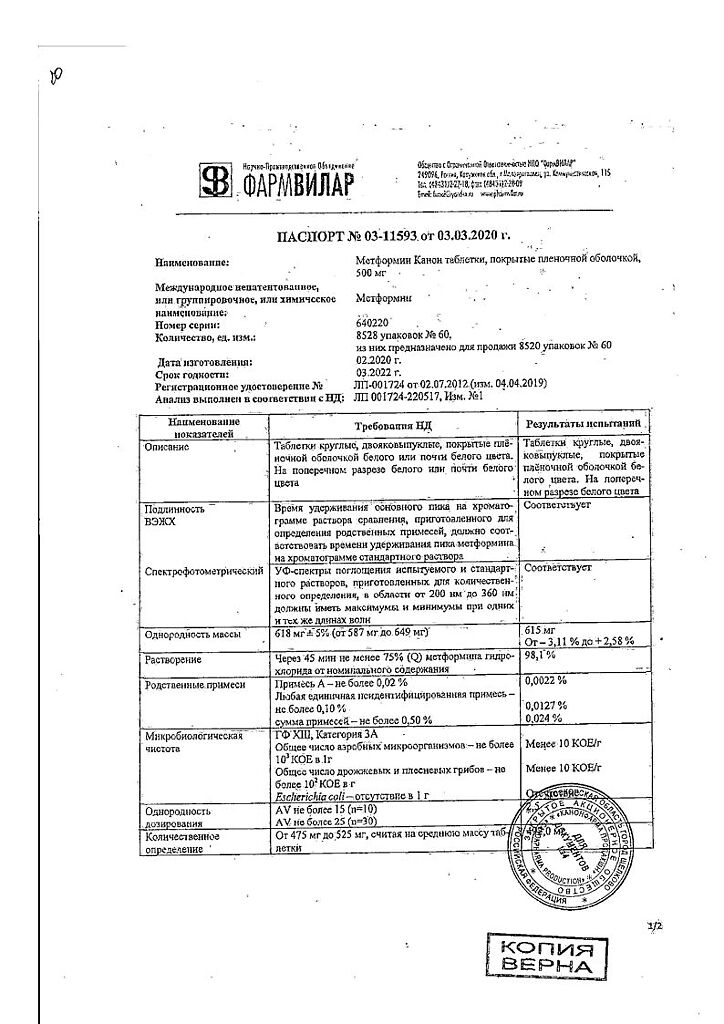
Metformin Canon, 500 mg 60 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Metformin inhibits gluconeogenesis in the liver, reduces glucose absorption from the intestine, increases peripheral glucose utilization, and increases tissue sensitivity to insulin.
With this it has no effect on insulin secretion by beta cells of the pancreas, does not cause hypoglycemic reactions. Reduces the level of triglycerides and low-density linoproteins in the blood. Stabilizes or reduces body weight. Has a fibrinolytic effect due to the inhibition of tissue-type plasminogen activator inhibitor.
PHARMACOKINETICS
After oral administration, metformin is absorbed from the GI tract. Bioavailability after a standard dose is 50-60%. Cmax in plasma is reached 2.5 hours after oral administration.
It practically does not bind with plasma proteins. It accumulates in salivary glands, muscles, liver and kidneys. It is excreted unchanged by kidneys. T1/2 is 9-12 hours. Cumulation of the preparation is possible in patients with impaired renal function.
Indications
Indications
Type 2 diabetes without predisposition to ketoacidosis (especially in obese patients) when diet therapy is ineffective; in combination with insulin, in type 2 diabetes, especially with a pronounced degree of obesity accompanied by secondary insulin resistance.
Active ingredient
Active ingredient
Composition
Composition
Active substances:
metformin 500 mg
Auxiliary substances:
povidone K 90;
corn starch;
crosspovidone;
magnesium stearate;
talc
Composition of the shell:
Methacrylic acid and methyl methacrylate copolymer (eudragit L 100-55); macrogol 6000; titanium dioxide; talc
How to take, the dosage
How to take, the dosage
The dose of the drug is set by the doctor individually, depending on the level of glucose in the blood.
The initial dose is 500-1000 mg per day (1-2 tablets). After 10-15 days further gradual increase of the dose depending on blood glucose level is possible.
The maintenance dose of the drug is usually 1500-2000 mg per day. (3-4 tablets) The maximum dose is 3000 mg per day (6 tablets).
In elderly patients, the recommended daily dose should not exceed 1 g (2 tablets).
The tablets Metformin should be taken whole during or immediately after a meal, with a small amount of liquid (glass of water). To reduce gastrointestinal side effects, the daily dose should be divided into 2 to 3 doses.
Because of the increased risk of lactoacidosis, the drug dose should be reduced if there are severe metabolic disorders.
Interaction
Interaction
The concomitant administration of danazol is not recommended to avoid the hyperglycemic effects of the latter. If treatment with danazolol is necessary and after discontinuation of the latter, metformin dose should be adjusted and glycemic levels monitored.
Combinations requiring special caution: Chlorpromazine – when taken in high doses (100 mg/day) increases glycemia by reducing insulin release.
When treated with neuroleptics and after discontinuation of the latter, metformin dose adjustment is required with glycemic control.
Concomitant use with sulfonylurea derivatives, acarbose, insulin, NSAIDs, MAO inhibitors, oxytetracycline, ACE inhibitors, clofibrate derivatives, cyclophosphamide, β-adrenoblockers may increase the hypoglycemic effect of metformin.
Concomitant use with GCS, oral contraceptives, epinephrine, sympathomimetics, glucagon, thyroid hormones, thiazide and loop diuretics, phenothiazine derivatives, nicotinic acid derivatives may decrease the hypoglycemic effect of metformin.
Cimetidine slows metformin excretion, which increases the risk of lactacidosis.
Metformin may weaken the effect of anticoagulants (coumarin derivatives).
Alcohol intake increases the risk of lactacidosis during acute alcohol intoxication, especially in cases of starvation or compliance with a low-fat diet, as well as in liver failure.
Special Instructions
Special Instructions
Key kidney function should be monitored during treatment. Plasma lactate should be determined at least twice a year, as well as in case of myalgia. In addition, serum creatinine level should be controlled once every 6 months (especially in elderly patients). Metformin should not be prescribed if blood creatinine level is higher than 135 μmol/l in men and 110 μmol/l in women.
Metformin may be used in combination with sulfonylurea derivatives. In this case, particularly careful monitoring of blood glucose levels is necessary.
For 48 hours before and for 48 hours after X-ray contrast (urography, IV angiography), Metformin should be discontinued.
If a patient has a bronchopulmonary infection or a urinary tract infection, inform the attending physician immediately.
Alcohol and medications containing ethanol should be avoided during treatment.
Impact on driving and operating machinery
The use of the drug in monotherapy does not affect the ability to drive and operate machinery.
When combining Metformin with other hypoglycemic agents (sulfonylurea derivatives, insulin) hypoglycemic states may occur, which impair the ability to drive vehicles and to engage in other potentially dangerous activities requiring increased attention and quick psychomotor reactions.
Contraindications
Contraindications
The drug is not recommended for persons older than 60 years of age who perform heavy physical work, due to the increased risk of lactic acidosis in them.
Side effects
Side effects
Gastrointestinal system disorders: nausea, vomiting, metallic taste in the mouth, lack of appetite, diarrhea, flatulence, abdominal pain. These symptoms are especially common at the beginning of treatment and usually go away on their own. These symptoms may be relieved by administration of antacids, atropine derivatives, or antispasmodics.
Metabolic disorders: in rare cases – lactacidosis (requires discontinuation of treatment); with long-term treatment – hypovitaminosis B12 (malabsorption).
Hematopoietic organs: in rare cases – megaloblastic anemia.
Endocrine system: hypoglycemia.
Allergic reactions: skin rash.
Overdose
Overdose
In case of overdose of Metformin drug, lactacidosis with fatal outcome is possible. The cause of lactacidosis may also be cumulation of the drug due to renal dysfunction.
The symptoms of lactacidosis: nausea, vomiting, diarrhea, decreased body temperature, abdominal pain, muscle pain; rapid breathing, dizziness, loss of consciousness and coma may occur.
Treatment: if signs of lactacidosis occur, treatment with Metformin should be stopped immediately, the patient should be immediately hospitalized and the lactate concentration determined to confirm the diagnosis. The most effective measure of lactate and metformin elimination from the body is hemodialysis. Symptomatic treatment is also carried out.
When combined therapy with sulfonylurea preparations with Metformin may lead to hypoglycemia.
Pregnancy use
Pregnancy use
If pregnancy is planned, and if pregnancy occurs while taking Metformin, it should be cancelled and insulin therapy should be prescribed.
As there are no data on penetration into breast milk, this drug is contraindicated during breastfeeding. If it is necessary to use Metformip during breastfeeding, breastfeeding should be stopped.
Similarities
Similarities
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 °C |
| Manufacturer | Kanonfarma Production ZAO, Russia |
| Medication form | pills |
| Brand | Kanonfarma Production ZAO |
Related products
Buy Metformin Canon, 500 mg 60 pcs with delivery to USA, UK, Europe and over 120 other countries.