No products in the cart.
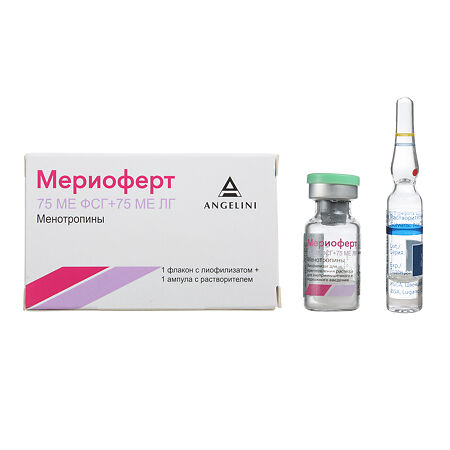
Meriofert, lyophilizate 75 me fsg+75 me lg
€1.00
Out of stock
(E-mail when Stock is available)
EAN: 7612291483818
SKU: 326219
Categories: Gynecology and Obstetrics, Infertility treatment IVF, Medicine
Description
Meriofert is a highly purified human menopausal gonadotropin (hMG) preparation. It belongs to the group of menotropins. The ratio of the biological activity of follicle stimulating hormone (FSH) and luteinizing hormone (LH) is 1:1. The drug is obtained from the urine of postmenopausal women.
The specific receptors for gonadotropins are present only in the tissues of the genital organs. In the ovaries, LH binds to receptors on the surface of theca cells and the corpus luteum, as well as to granulosa cells of large follicles.
FSH binds to receptors on the surface of granulosa cells of small follicles in the ovaries and Sertoli cells in the testes.
In women, Meriofert stimulates growth and maturation of ovarian follicles, increases the concentration of estrogens, and stimulates endometrial proliferation. In men, it stimulates spermatogenesis in azoospermia and oligoasthenospermia.
Indications
Indications
For women:
· Anovulation (including polycystic ovary syndrome (PCOS) when clomiphene therapy is ineffective).
· Controlled ovarian hyperstimulation to induce the growth of multiple follicles during assisted reproductive technologies (ART).
For men:
· Stimulation of spermatogenesis in azoospermia and oligoasthenospermia caused by congenital or acquired hypogonadotropic hypogonadism (in combination with the drug human chorionic gonadotropin (hCG)).
Pharmacological effect
Pharmacological effect
The drug Meriofert is a highly purified human menopausal gonadotropin (hMG) drug. Belongs to the group of menotropins. The ratio of the biological activity of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) is 1:1. The drug is obtained from the urine of postmenopausal women.
Specific receptors for gonadotropins are present only in the tissues of the genital organs. In the ovaries, LH binds to receptors on the surface of the theca cells and the corpus luteum, as well as to granulosa cells of large follicles.
FSH binds to receptors on the surface of granulosa cells of small follicles in the ovaries and Sertoli cells in the testes.
In women, the drug Meriofert stimulates the growth and maturation of ovarian follicles, increases the concentration of estrogen, and stimulates endometrial proliferation. In men, it stimulates spermatogenesis in azoospermia and oligoasthenospermia.
Special instructions
Special instructions
Treatment should be carried out under the supervision of a doctor experienced in treating infertility.
Before starting to use the drug Meriofert, it is recommended to conduct an examination to identify hypothyroidism, adrenal insufficiency, hyperprolactinemia, tumors of the pituitary gland or hypothalamus; as well as appropriate specific treatment.
OHSS
OHSS is a syndrome distinct from uncomplicated ovarian enlargement, the manifestations of which depend on the severity. It includes significant ovarian enlargement, high serum estrogen concentrations, and increased vascular permeability, which can lead to fluid accumulation in the abdominal, pleural, and, rarely, pericardial cavities (in severe OHSS). In moderate OHSS, symptoms include abdominal pain, bloating, significant ovarian enlargement, weight gain, shortness of breath, oliguria, and gastrointestinal symptoms including nausea, vomiting, and diarrhea.
In severe OHSS, hypovolemia, hemoconcentration, electrolyte disturbances, ascites, hemoperitoneum, hydrothorax, acute respiratory distress syndrome and thromboembolic complications develop.
Excessive ovarian response to gonadotropin administration rarely leads to the development of OHSS unless hCG is administered to stimulate ovulation. Therefore, in case of ovarian hyperstimulation, hCG should not be administered, and the patient should be warned to abstain from sexual intercourse or use barrier methods of contraception for at least 4 days.
OHSS can progress rapidly (over 24 hours to several days) to become a serious medical complication, so patients should be monitored for at least 2 weeks after hCG administration. Compliance with the recommended doses of Meriofert, the administration regimen and careful monitoring of therapy can minimize cases of ovarian hyperstimulation and multiple pregnancies. When performing ART, aspiration of the contents of all follicles before ovulation can reduce the risk of developing OHSS.
OHSS may be more severe and protracted as pregnancy progresses. Most often, OHSS develops after cessation of treatment with gonadotropins and reaches maximum severity within 7-10 days after the end of treatment. OHSS usually resolves spontaneously after the onset of menstruation.
For moderate to severe OHSS, the patient is hospitalized and specific therapy is started.
OHSS occurs with high frequency in patients with polycystic ovary syndrome.
Multiple pregnancy.
Multiple pregnancies are associated with an increased risk of adverse maternal and perinatal outcomes. When using menotropins, multiple pregnancies develop more often than with natural conception. In the case of in vitro fertilization (IVF), the likelihood of a multiple pregnancy depends on the number of embryos introduced, their quality and the age of the patient. The patient should be warned about the potential risk of multiple pregnancies before starting treatment.
Complications of pregnancy.
The frequency of spontaneous abortions during pregnancy occurring after treatment with Meriofert is higher than in healthy women.
Ectopic pregnancy.
With a history of fallopian tube diseases, both during natural conception and during infertility treatment, women have a high risk of ectopic pregnancy. The prevalence of ectopic pregnancy after IVF is 2 to 5%, compared to 1 to 1.5% in the general population.
Neoplasms of the reproductive system.
There are reports of neoplasms of the ovaries and other organs of the reproductive system, both benign and malignant, in women who have undergone infertility treatment using several ART techniques. It has not yet been established whether treatment with gonadotropins increases the baseline risk of these tumors in infertile women.
Congenital defects
The prevalence of congenital malformations of the fetus using ART is slightly higher than with natural conception. It is believed that this may be due to the individual characteristics of the parents (mother’s age, sperm characteristics) and multiple pregnancies.
Thromboembolic complications
Women with known risk factors for thromboembolic complications, such as personal or family history, obesity (body mass index > 30 kg/m2) or thrombophilia, may have an increased risk of venous or arterial thromboembolic complications during or after treatment with gonadotropins. In such cases, the benefit of their use must outweigh the risk. It should be borne in mind that pregnancy itself also increases the risk of thromboembolic complications.
In men with a high concentration of FSH in the blood (indicating primary hypogonadism), Meriofert is usually ineffective.
Impact on the ability to drive vehicles and machinery
The drug Meriofert does not affect the ability to drive vehicles and machines.
Active ingredient
Active ingredient
Menotropins
Composition
Composition
1 bottle of lyophilisate contains:
Active ingredient: highly purified human menopausal gonadotropin hMG (menotropin) 75 IU FSH + 75 IU LH, 150 IU FSH + 150 IU LH
Auxiliary component – lactose monohydrate 10.00 mg
Contraindications
Contraindications
· Hypersensitivity to menotropin and other components of the drug.
Tumors of the pituitary gland or hypothalamus.
· Decompensated thyroid diseases, adrenal insufficiency, hyperprolactinemia.
· Age up to 18 years.
For women:
· Persistent enlargement of the ovaries, ovarian cysts (not caused by polycystic ovary syndrome).
· Anomalies in the development of the genital organs, incompatible with pregnancy.
· Uterine fibroids, incompatible with pregnancy.
· Bleeding from the vagina of unknown etiology.
· Estrogen-dependent tumors (ovarian cancer, uterine cancer or breast cancer).
· Primary ovarian failure.
· Pregnancy and breastfeeding period.
For men:
· Prostate cancer, testicular tumor, androgen-dependent tumors.
· Primary testicular failure.
With caution
The presence of risk factors for thromboembolic complications, such as individual or family predisposition, severe obesity (body mass index >30 kg/m2) or thrombophilia, since in this case there is an increased risk of developing venous or arterial thrombosis and thromboembolism during or after the use of gonadotropins. In this case, the benefits of treatment with gonadotropins should outweigh the risks of their use.
Use during pregnancy and breastfeeding The drug is contraindicated during pregnancy and breastfeeding.
Side Effects
Side Effects
Undesirable side effects observed when taking the drug were in most cases moderate and transient.
The most common adverse reactions were: ovarian cyst formation, injection site reactions and headache (with an incidence of up to 10%). The most serious adverse reactions were OHSS and complications associated with this syndrome.
The main adverse reactions are listed in the table below:
Adverse adverse reactions were classified as follows:
very common (>1/10); frequent – (from > 1/100 to 1/1000 to <
1/100); rare – (from > 1/10000 to < 1/1000); very rare - (< 1/10000).
Organs and systems
Frequency
Adverse reaction
From the gastrointestinal tract
Often
Gastrointestinal syndrome, including nausea, vomiting, diarrhea, intestinal colic and bloating, abdominal pain
General and administration site disorders
Very often
Pain, redness, bruising, swelling and/or irritation at the injection site
From the nervous system
Very often
Headache
Disorders of the genital organs and breast
Very often
Often
Rarely
Ovarian enlargement and formation of ovarian cysts
Ovarian hyperstimulation syndrome, gynecomastia (in men)
Ovarian torsion
From the cardiovascular system
Very rarely
Thromboembolism
From the immune system
Very rarely
Systemic allergic reactions such as skin redness and skin rash, angioedema
From the skin and subcutaneous tissue (in men)
Often
Acne
Interaction
Interaction
Pharmacodynamic
The drug Meriofert can be prescribed alone or in combination with agonists or antagonists of gonadotropin releasing hormone (GnRH)
The combined use of Meriofert with other drugs used to stimulate ovulation (for example, hCG, clomiphene) may enhance the follicular response. When used together with GnRH agonists, an increase in the dose of menotropin may be required.
Pharmaceutical
Meriofert should not be mixed with other medications in the same syringe.
Overdose
Overdose
In case of overdose, the development of OHSS and thromboembolic complications is possible. Symptoms of OHSS are ovarian enlargement, lower abdominal pain, nausea, vomiting, diarrhea, weight gain, oliguria, ascites, hydrothorax, hemoperitoneum, hemoconcentration, shortness of breath (detailed information is provided in the “Special Instructions” section).
Symptoms of mild to moderate OHSS usually do not require additional treatment and resolve on their own within 2-3 weeks.
In case of severe OHSS, hospitalization in the intensive care units of specialized gynecological hospitals is necessary for complex treatment.
Recommendations for use
Recommendations for use
The drug Meriofert is administered intramuscularly or subcutaneously with periodic changes in the injection site. The subcutaneous route of administration is preferable, as it ensures the greatest absorption of the drug. Therapy with Meriofert should only be carried out under the supervision of a physician with appropriate specialization and experience in the treatment of infertility.
The solution is prepared immediately before injection using the supplied solvent. The doses of the drug described below are given according to FSH and are the same for both subcutaneous and intramuscular routes of administration.
In women, the dose of the drug is set individually depending on the reaction of the ovaries. This requires monitoring the ovarian response to therapy in the form of ultrasound (ultrasound) in combination with determining the concentration of estradiol in the blood plasma.
Anovulation (including PCOS if clomiphene therapy is ineffective).
The drug Meriofert can be administered daily. The use of the drug should begin during the first 7 days of the menstrual cycle. The recommended initial dose is 75-150 IU/day. In the absence of a sufficient ovarian response, the dose is gradually increased. The recommended interval for increasing the dose should be at least 7 days. The recommended increasing dose is 37.5 IU, but not more than 75 IU.
The maximum daily dose usually does not exceed 225 IU. If the therapeutic effect is not achieved within 4 weeks of treatment, Meriofert injections are stopped and then a new cycle is started with a higher dose of the drug. The patient is recommended to use barrier methods of contraception or abstain from sexual intercourse until menstruation occurs.
When an adequate ovarian response is achieved, the next day after the last injection of the drug Meriofert, 5000-10000 IU of hCG is administered once to induce ovulation. The patient is recommended to have sexual intercourse on the day of hCG administration and the day after administration. As an alternative method, intrauterine insemination is possible.
If the ovaries react excessively, the administration of Meriofert should be stopped and the administration of hCG should be discontinued.
Treatment should be resumed in the next cycle at a lower dose than in the previous cycle.
Controlled ovarian hyperstimulation to induce the growth of multiple follicles during assisted reproductive technologies (BPT).
A widely used protocol for hyperstimulation involves the administration of 150-225 IU of Meriofert daily, starting on days 2 or 3 of the menstrual cycle and continuing until sufficient follicle size is achieved. The daily dose is adjusted according to the patient’s response to therapy. The daily dose of the drug should not exceed 450 IU FSH.
24-48 hours after the last injection of the drug Meriofert, one injection of hCG is prescribed at a dose of 5000 IU-10000 IU to induce the final maturation of the follicles.
To prevent the release of endogenous LH, gonadotropin releasing hormone agonists (GnRH agonists) are now widely used. In this case, the use of Meriofert should begin approximately two weeks after the start of treatment with GnRH agonists. In the future, both drugs continue to be used together until an adequate level of follicular development is achieved. The dose of Meriofert is adjusted according to the response of the patient’s ovaries.
For hypogonadotropic hypogonadism in men, Meriofert is prescribed to stimulate spermatogenesis if previous therapy with hCG drugs caused only an androgenic reaction without signs of increased spermatogenesis. In this case, treatment continues by administering 2000 IU of hCG 2 – 3 times a week along with injections of the drug Meriofert (75 IU or 150 IU) 2 – 3 times a week. Treatment with this regimen should be continued for at least 3 months.
If there is no positive effect during this time, treatment can be continued for up to 18 months.
Functional features
Functional features
The biological effectiveness of hMG is mainly due to its follicle-stimulating component. The pharmacokinetic properties of hMG when administered intramuscularly and subcutaneously have high individual variability.
Suction
According to studies conducted for the drug Meriofert, after a single subcutaneous and intramuscular administration of the drug at a dose of 300 IU, the time to reach the maximum concentration of the drug in plasma (Tmax) is 22 and 19 hours, respectively.
The bioavailability of the drug when administered subcutaneously is higher than when administered intramuscularly.
Removal
It is excreted primarily by the kidneys. The half-life of a single administration of the drug Meriofert (300 IU according to FSH) is approximately 45 hours for intramuscular administration and 40 hours for subcutaneous administration.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25 ° C in a place protected from light. The reconstituted solution should be used immediately.
Keep out of the reach of children.
Shelf life
Shelf life
2 years.
The shelf life of the kit is determined by the component with the shortest shelf life.
Do not use after expiration date.
Manufacturer
Manufacturer
IBSA Institute Biokimik S.A., Switzerland
Additional information
| Shelf life | 2 years. The expiration date of the kit is determined by the component with the lowest expiration date. Do not use after the expiration date. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25°C in a light-protected place. The reconstituted solution should be used immediately. Store out of the reach of children. |
| Manufacturer | IBSA Institute Biokimik S.A., Switzerland |
| Medication form | lyophilizate |
| Brand | IBSA Institute Biokimik S.A. |
Related products
Gynecology and Obstetrics
Gynecology and Obstetrics
Prepidil, intracervical gel 0.5 mg/3 g syringes with catheter
Buy Meriofert, lyophilizate 75 me fsg+75 me lg with delivery to USA, UK, Europe and over 120 other countries.