No products in the cart.
Magnelis B6 forte, 100 mg+10 mg 60 pcs
€20.34 €16.95
Description
Pharmacotherapeutic group: magnesium drug.
TAC code: A12CC
Pharmacological properties
Pharmacodynamics
Magnesium is a vital element that is necessary for normal cell function, it participates in most metabolic reactions. In particular it participates in the regulation of nerve impulse transmission and muscle contraction. 50% of the amount of magnesium contained in the body is accumulated in bone tissue.
Pyridoxine (vitamin B6) participates in many metabolic processes, improves the absorption of magnesium from the gastrointestinal tract and its penetration into cells.
Serum magnesium concentrations:
in the range 12 and17 mg/L (1 – 1.4 mEq/L or 0.5 – 0.7 mmol/L): Indicates moderate magnesium deficiency;
below 12 mg/L (1 mEq/L or 0.5 mmol/L): indicates severe magnesium deficiency.
The body gets magnesium with food. Magnesium deficiency in the body can occur when there is a deficiency in intake or when the need for magnesium increases.
Pharmacokinetics
The gastrointestinal absorption of magnesium salts occurs in part by a passive mechanism in which salt solubility plays a determining role. The degree of this absorption does not exceed 50%. Excretion occurs mainly by the kidneys.
Additional information
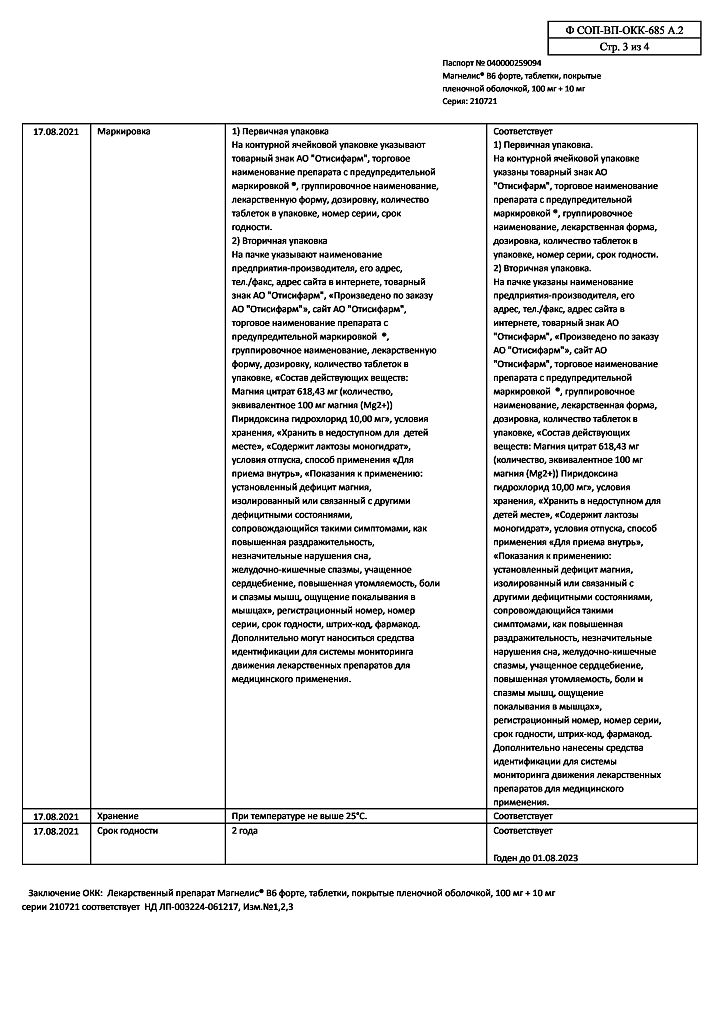
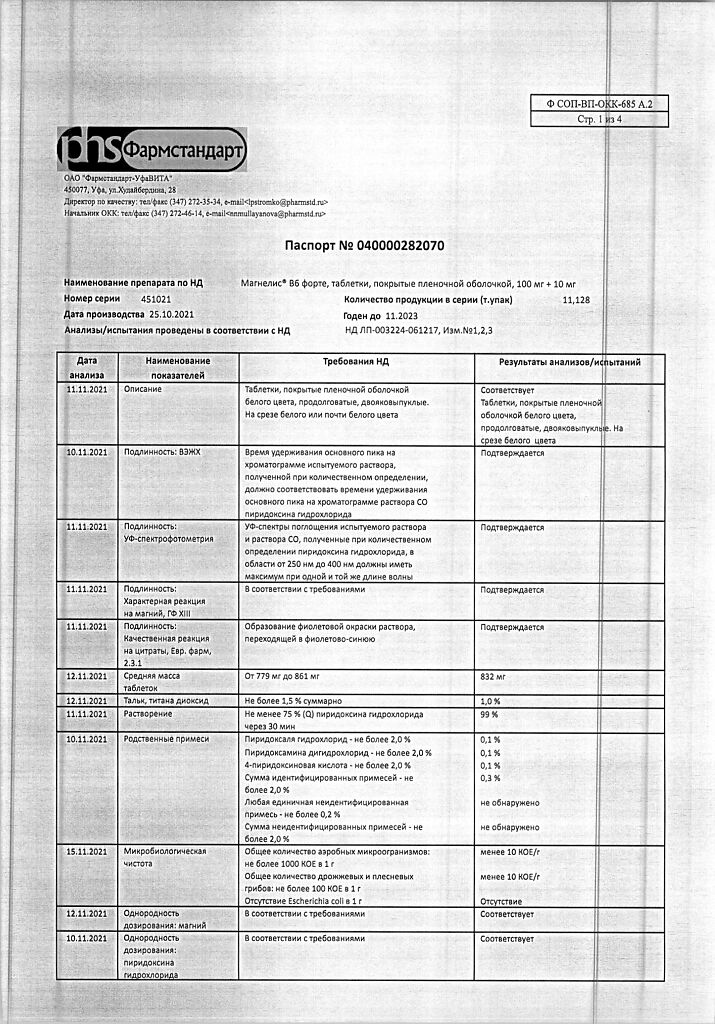
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | At a temperature not higher than 25 ° C. Keep out of reach of children. |
| Manufacturer | Pharmstandard-UfaVITA, Russia |
| Medication form | pills |
| Brand | Pharmstandard-UfaVITA |
Related products
Buy Magnelis B6 forte, 100 mg+10 mg 60 pcs with delivery to USA, UK, Europe and over 120 other countries.