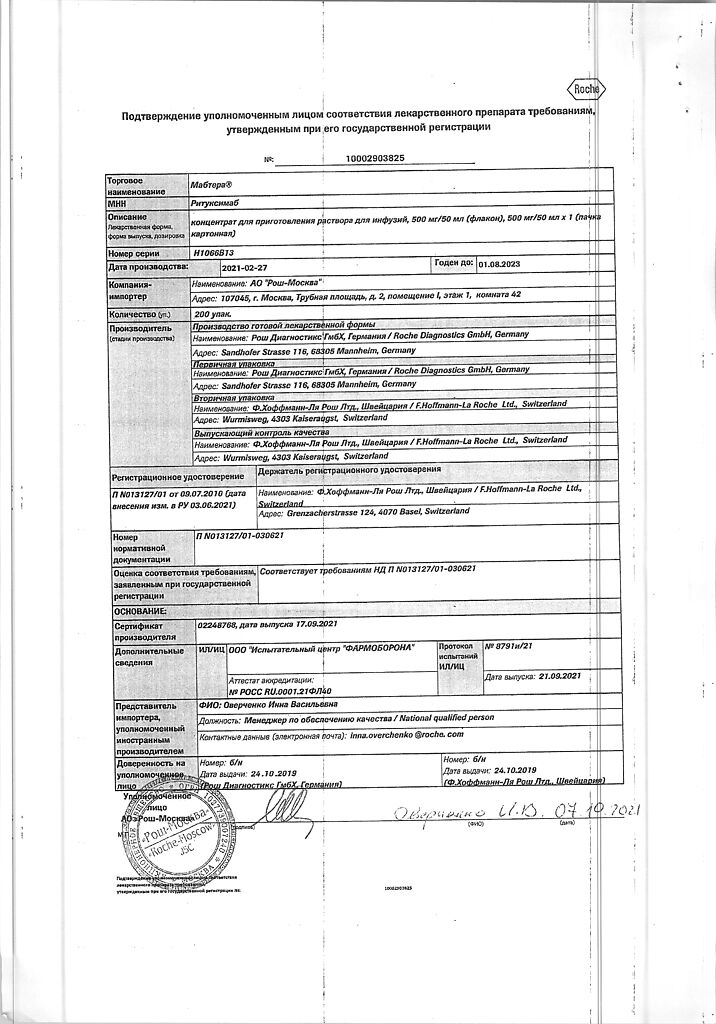
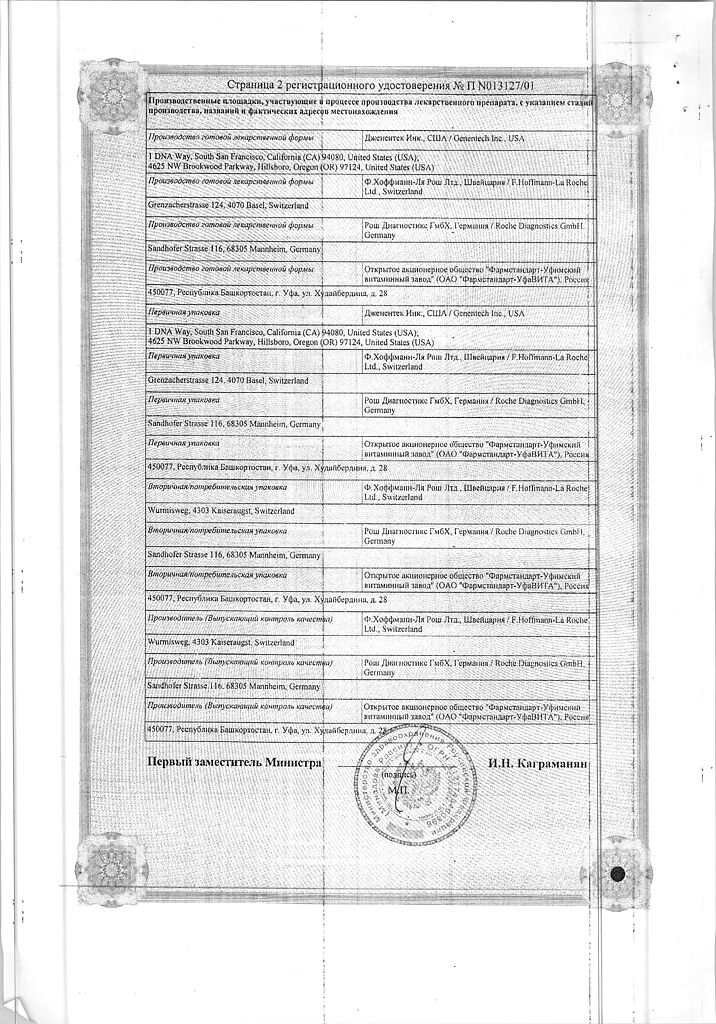
No products in the cart.
Description
Pharmacodynamics
Antitumor and immunomodulatory drug. Rituximab is a chimeric mouse/human monoclonal antibody that binds specifically to the transmembrane antigen CD20. This antigen is located on pre-B-lymphocytes and mature B-lymphocytes, but is absent on hematopoietic stem cells, pro-B cells, normal plasma cells, cells of other tissues and is expressed in more than 95% of B-cell non-Hodgkin lymphomas. Cell-expressed CD20 is not internalized after binding to the antibody and is no longer transported from the cell membrane into the extracellular space. CD20 does not circulate in the plasma as a free antigen and therefore does not compete for binding to the antibody.
Rituximab binds to the CD20 antigen on B-lymphocytes and initiates immunological responses mediating B-cell lysis. Possible mechanisms of cell lysis include complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity, and apoptosis induction. Rituximab increases the sensitivity of human B-cell lymphoma cell lines to the cytotoxic effects of certain chemotherapeutic agents in vitro.
The number of B-cells in peripheral blood decreases below normal after the first administration of the drug and begins to recover in patients with hematologic malignancies after 6 months, reaching normal values 12 months after completion of therapy, but in some cases the duration of B-cell recovery may be longer.
In patients with rheumatoid arthritis, the duration of decline in B-cell counts varies, with most patients receiving follow-up therapy until their numbers are fully restored. In a small number of patients there is a long-term decrease in the number of B-cells (for 2 years or more after the last dose of therapy).
In patients with granulomatosis with polyangiitis and microscopic polyangiitis, a decrease in CD19-positive B-cells to less than 10 cells/μL occurs after the first two infusions of rituximab and in most patients remains at this level for 6 months.
Antichimeric antibodies were detected in 1.1% of patients with non-Hodgkin’s lymphoma and in 10% of patients with rheumatoid arthritis. Anti-mouse antibodies were not detected in the examined patients.
Pharmacokinetics
Non-Hodgkin’s lymphoma
. According to population pharmacokinetic analysis in patients with non-Hodgkin’s lymphoma when administered once or multiple times with Mabthera® as monotherapy or in combination with chemotherapy according to the CHOP regimen (cyclosporine, doxorubicin, vincristine, prednisolone) non-specific clearance (CL1), specific clearance (CL2), (probably related to B-cells or tumor burden), and plasma volume distribution (V1) are 0.14 L/day, 0.59 L/day and 2.7 L respectively. The median terminal T1/2 is 22 days. The baseline level of CD19-positive cells and tumor site size affect the CL2 of rituximab 375 mg/m2 v/v once weekly, for 4 weeks. CL2 was higher in patients with higher levels of CD19-positive cells or larger tumor nidus size. Individual variability in CL2 persisted even after correction of tumor focus size and CD19-positive cell levels. Relatively small changes in V1 depend on body surface area (1.53-2.32 m2) and on CHOP chemotherapy and are 27.1% and 19%, respectively. Age, sex, race, and general health according to the WHO scale have no effect on rituximab pharmacokinetics. Thus, adjusting the dose of rituximab according to the factors listed above does not significantly affect pharmacokinetic variability.
The mean Cmax increases after each infusion: 243 µg/ml after the first infusion, 486 µg/ml after the fourth infusion, and 550 µg/ml after the eighth infusion. Cmin and Cmax of the drug inversely correlate with the initial number of CD19-positive B-cells and tumor burden. With effective treatment, the median Css of the drug is higher. The median Css of the drug is higher in patients with histological subtypes of tumor B, C and D (IWF classification – International Working Formulation) than with subtype A. Traces of rituximab can be detected in the body for 3-6 months after the last infusion.
The pharmacokinetic profile of rituximab (6 infusions of 375 mg/m2) in combination with 6 cycles of SNR chemotherapy was almost identical to that of monotherapy.
Cronic lympholeukemia
The mean Cmax after the fifth infusion of rituximab at a dose of 500 mg/m2 is 408 mcg/mL.
Rheumatoid arthritis
After two 1,000-mg infusions by IV with a 2-week break, the mean Cmax of rituximab is 369 mcg/mL, mean T1/2 is 19.2-20.8 days, mean systemic clearance is 0.23 L/day and Vd in equilibrium is 4.6 L. After the second infusion, the mean Cmax was 16-19% higher compared to the first infusion. On retreatment, the pharmacokinetic parameters of rituximab are comparable to the first course of treatment.
Granulomatosis with polyangiitis (Wegener’s granulomatosis) and microscopic polyangiitis
After four infusions of rituximab at 375 mg/m2 once weekly, median T1/2 was 23 days, mean clearance was 0.313 L/day and Vd was 4.5 L, based on population pharmacokinetic analysis. The pharmacokinetic parameters of rituximab in granulomatosis with polyangiitis and microscopic polyangiitis were almost the same as in rheumatoid arthritis.
Pharmacokinetics in selected patient groups
The Vd and clearance of rituximab adjusted for body surface area are slightly greater in men than in women; no dose adjustment of rituximab is required. No pharmacokinetic data are available in patients with renal and hepatic impairment.
Indications
Indications
Non-Hodgkin’s lymphoma:
relapsed or chemoresistant B-cell, CD20-positive low-grade or follicular non-Hodgkin lymphoma;
stage III-IV follicular lymphoma in combination with chemotherapy in previously untreated patients;
follicular lymphoma as maintenance therapy after response to induction therapy;
CD20-positive diffuse large B-cell non-Hodgkin lymphoma in combination with CHOP chemotherapy.
Chronic lymphocytic leukemia:
chronic lymphocytic leukemia in combination with chemotherapy in patients who have not previously received standard therapy;
relapsing or chemotherapy-resistant chronic lymphocytic leukemia in combination with chemotherapy.
Rheumatoid arthritis:
moderate to severe rheumatoid arthritis (active form) in adults in combination with methotrexate with intolerance or inadequate response to current treatment regimens that include one or more TNF-α inhibitors, incl. to inhibit radiographically proven joint destruction.
Granulomatosis with polyangiitis (Wegener’s granulomatosis) and microscopic polyangiitis:
severe forms of active granulomatosis with polyangiitis (Wegener’s granulomatosis) and microscopic polyangiitis in combination with GCS.
Pharmacological effect
Pharmacological effect
Pharmacodynamics
Antitumor and immunomodulatory drug. Rituximab is a mouse/human chimeric monoclonal antibody that specifically binds to the CD20 transmembrane antigen. This antigen is located on pre-B lymphocytes and mature B lymphocytes, but is absent on hematopoietic stem cells, pro-B cells, normal plasma cells, and cells of other tissues and is expressed in more than 95% of cases of B-cell non-Hodgkin lymphoma. CD20 expressed on the cell after binding to the antibody is not internalized and ceases to flow from the cell membrane into the extracellular space. CD20 does not circulate in plasma as a free antigen and therefore does not compete for antibody binding.
Rituximab binds to the CD20 antigen on B cells and initiates immunological responses that mediate B cell lysis. Possible mechanisms of cell lysis include complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity, and induction of apoptosis. Rituximab sensitizes human B-cell lymphoma lines to the cytotoxic effects of certain chemotherapeutic drugs in vitro.
The number of B cells in the peripheral blood after the first administration of the drug decreases below normal and begins to recover in patients with hematological malignancies after 6 months, reaching normal values 12 months after completion of therapy, but in some cases the duration of the period of recovery of the number of B cells may be longer.
In patients with rheumatoid arthritis, the duration of B cell decline varies, and most patients are given follow-up therapy until their numbers are fully restored. A small number of patients experience a long-term decline in B cell counts (for 2 years or more after the last dose of the drug).
In patients with granulomatosis with polyangiitis and microscopic polyangiitis, a decrease in the number of CD19-positive B cells to less than 10 cells/μl occurs after the first two infusions of rituximab and in most patients remains at this level for 6 months.
Antichimeric antibodies were detected in 1.1% of examined patients with non-Hodgkin’s lymphoma and in 10% with rheumatoid arthritis. Anti-mouse antibodies were not detected in the examined patients.
Pharmacokinetics
Non-Hodgkin’s lymphoma
Based on a population pharmacokinetic analysis in patients with non-Hodgkin’s lymphoma, with single or multiple doses of MabThera as monotherapy or in combination with CHOP chemotherapy (cyclosporine, doxorubicin, vincristine, prednisolone), nonspecific clearance (CL1), specific clearance (CL2), (likely related to B cells or tumor load), and volume of distribution in plasma (V1) are 0.14 l/day, 0.59 l/day and 2.7 l, respectively. The median terminal T1/2 is 22 days. The initial level of CD19-positive cells and the size of the tumor lesion affect the CL2 of rituximab 375 mg/m2 IV once a week for 4 weeks. The CL2 score is higher in patients with a higher level of CD19-positive cells or a larger tumor size. Individual variability in CL2 persists after correction for tumor size and the level of CD19-positive cells. Relatively small changes in V1 depend on the size of the body surface area (1.53-2.32 m2) and on CHOP chemotherapy and amount to 27.1% and 19%, respectively. Age, gender, race, and general condition according to the WHO scale do not affect the pharmacokinetics of rituximab. Thus, dose adjustment of rituximab depending on the above factors does not significantly affect pharmacokinetic variability.
The average Cmax increases after each infusion: after the first infusion – 243 mcg/ml, after the fourth infusion – 486 mcg/ml, after the eighth – 550 mcg/ml. Cmin and Cmax of the drug are inversely correlated with the initial number of CD19-positive B cells and the magnitude of the tumor load. With effective treatment, the median Css of the drug is higher. The median Css of the drug is higher in patients with histological tumor subtypes B, C and D (IWF – International Working Formulation classification) than with subtype A. Traces of rituximab can be detected in the body for 3-6 months after the last infusion.
The pharmacokinetic profile of rituximab (6 infusions of 375 mg/m2) in combination with 6 cycles of CHOP chemotherapy was almost the same as with monotherapy.
Chronic lymphocytic leukemia
The average Cmax after the fifth infusion of rituximab at a dose of 500 mg/m2 is 408 mcg/ml.
Rheumatoid arthritis
After two intravenous infusions of 1000 mg with a 2-week break, the average Cmax of rituximab was 369 mcg/ml, the average T1/2 was 19.2-20.8 days, the average systemic clearance was 0.23 l/day and Vd at steady state was 4.6 l. After the second infusion, the average Cmax is 16-19% higher compared to the first infusion. When a second course of treatment is carried out, the pharmacokinetic parameters of rituximab are comparable to the first course of treatment.
Granulomatosis with polyangiitis (Wegener’s granulomatosis) and microscopic polyangiitis
According to a population pharmacokinetic analysis, after four infusions of rituximab at a dose of 375 mg/m2 once a week, the median T1/2 was 23 days, the average clearance was 0.313 l/day and Vd was 4.5 l. The pharmacokinetic parameters of rituximab in granulomatosis with polyangiitis and microscopic polyangiitis were almost the same as in rheumatoid arthritis.
Pharmacokinetics in selected patient groups
Vd and clearance of rituximab adjusted for body surface area in men are slightly greater than in women; no dose adjustment of rituximab is required. Pharmacokinetic data in patients with renal and hepatic insufficiency are not available.
Special instructions
Special instructions
The patient’s medical documentation should indicate the trade name of the drug (MabThera®). Replacing the drug with any other biological drug requires agreement with the attending physician. The information provided in this leaflet applies only to MabThera®.
MabThera® is administered under the close supervision of an oncologist, hematologist or rheumatologist if the necessary conditions for resuscitation are available.
Non-Hodgkin’s lymphoma and chronic lymphocytic leukemia
Infusion reactions. The development of infusion reactions may be due to the release of cytokines and/or other mediators. Severe infusion reactions are difficult to distinguish from hypersensitivity reactions or cytokine release syndrome. There are reports of fatal infusion reactions described during post-marketing use of the drug. Most patients develop fever with chills or trembling within 0.5-2 hours after the start of the first infusion of MabThera®.
Severe reactions include pulmonary symptoms, low blood pressure, urticaria, angioedema, nausea, vomiting, weakness, headache, itching, tongue irritation or pharyngeal swelling (vascular edema), rhinitis, hot flashes, focal pain and, in some cases, signs of rapid tumor lysis syndrome. Infusion reactions disappear after interrupting the administration of MabThera® and drug therapy (including intravenous administration of 0.9% sodium chloride solution, diphenhydramine and acetaminophen, bronchodilators, corticosteroids, etc.). In most cases, after complete resolution of symptoms, the infusion can be resumed at a rate of 50% of the previous rate (eg, 50 mg/hour instead of 100 mg/hour).
In the majority of patients with non-life-threatening infusion reactions, the course of treatment with rituximab was completely completed. Continuation of therapy after complete disappearance of symptoms is rarely accompanied by recurrence of severe infusion reactions.
Due to the potential for the development of anaphylactic reactions and other hypersensitivity reactions with intravenous administration of protein drugs, it is necessary to have means to relieve them: adrenaline, antihistamines and corticosteroids.
Side effects from the lungs. Hypoxia, pulmonary infiltrates and acute respiratory failure. Some of these events were preceded by severe bronchospasm and dyspnea. Symptoms may worsen over time or clinical deterioration may occur after initial improvement. Patients with pulmonary symptoms or other severe infusion reactions should be closely monitored until symptoms resolve completely. Acute respiratory failure may be accompanied by the formation of interstitial infiltrates in the lungs or pulmonary edema, often manifesting itself in the first 1-2 hours after the start of the first infusion. If severe reactions from the lungs develop, the rituximab infusion should be stopped immediately and intensive symptomatic therapy should be prescribed. Since initial improvement in clinical symptoms may be followed by worsening, patients should be carefully monitored until pulmonary symptoms resolve.
Rapid tumor lysis syndrome. MabThera® mediates rapid lysis of benign or malignant CD20-positive cells. Tumor lysis syndrome is possible after the first infusion of MabThera® in patients with a large number of circulating malignant lymphocytes. Tumor lysis syndrome includes: hyperuricemia, hyperkalemia, hypocalcemia, hyperphosphatemia, acute renal failure, increased LDH activity. Patients at risk (patients with a high tumor burden or with a circulating malignant cell count >25,000/mm3, such as chronic lymphocytic leukemia or mantle cell lymphoma) require close medical supervision and regular laboratory testing. If symptoms of rapid tumor lysis develop, appropriate therapy is administered. After complete relief of symptoms in a limited number of cases, MabThera therapy was continued in combination with rapid tumor lysis syndrome prophylaxis.
In patients with a high number of circulating malignant cells (>25,000/μl) or a high tumor burden (eg, chronic lymphocytic leukemia or mantle cell lymphoma), in whom the risk of extremely severe infusion reactions may be particularly high, MabThera should be administered with extreme caution and close monitoring. The first infusion of the drug in such patients should be administered at a slower rate or divided into 2 days during the first cycle of therapy and each subsequent cycle if the number of circulating malignant cells remains >25,000/mm3.
Side effects from the cardiovascular system. During infusion, careful monitoring of patients with a history of cardiovascular disease is required due to the possibility of developing angina, arrhythmia (atrial flutter and fibrillation), heart failure or myocardial infarction. Due to the possibility of hypotension, antihypertensive medications should be discontinued at least 12 hours before MabThera infusion.
Control of blood cells. Although monotherapy with MabThera does not have a myelosuppressive effect, caution should be exercised when prescribing the drug for neutropenia less than 1500/μL and/or thrombocytopenia less than 75,000/μL, since the experience of its clinical use in such patients is limited. MabThera® has been used in patients after autologous bone marrow transplantation and in other risk groups with possible bone marrow dysfunction without causing myelotoxicity. During treatment, it is necessary to regularly determine a detailed peripheral blood count, including platelet count, in accordance with routine practice.
Infections. MabThera should not be used in patients with severe acute infection.
Hepatitis B. When prescribing a combination of MabThera® with chemotherapy, reactivation of the hepatitis B virus or fulminant hepatitis (including with a fatal outcome) was observed. Predisposing factors included both the stage of the underlying disease and cytotoxic chemotherapy.
Before prescribing MabThera®, all patients should be screened for hepatitis B. The minimum set of tests should include determination of HBsAg and HBcAb, in accordance with local recommendations, the list of tests can be expanded. MabThera® should not be used in patients with active hepatitis B. Patients with positive serological markers for hepatitis B should consult a hepatologist before using MabThera®; For such patients, appropriate monitoring and measures should be taken to prevent hepatitis B virus reactivation in accordance with local standards.
Progressive multifocal leukoencephalopathy (PML). Cases of PML have been observed with MabThera® use in patients with non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Most patients received MabThera in combination with chemotherapy or in combination with hematopoietic stem cell transplantation. If neurological symptoms occur in such patients, it is necessary to conduct a differential diagnosis to exclude PML and consult a neurologist.
Skin reactions. Cases of the development of such severe skin reactions as toxic epidermal necrolysis and Stevens-Johnson syndrome, in some cases with a fatal outcome, have been reported. If these reactions are detected, MabThera® should be discontinued.
Immunization. The safety and effectiveness of immunization with live viral vaccines following treatment with MabThera® has not been studied. Vaccination with live viral vaccines is not recommended. Vaccination with inactivated vaccines is possible, but response rates may be reduced. In patients with relapsed low-grade non-Hodgkin’s lymphoma, there was a decreased response rate to tetanus toxoid and KHL neoantigen (KHL hemocyanin fissurelia) compared with patients not receiving MabThera (16% vs. 81% and 4% vs. 76%, respectively; end point was a greater than 2-fold increase in antibody titer). However, the average antibody titer to a set of antigens (Streptococcus pneumoniae, influenza A, mumps, rubella, chickenpox) did not change for at least 6 months after treatment with MabThera® (when compared with the antibody titer before treatment).
Rheumatoid arthritis, granulomatosis with polyangiitis (Wegener’s granulomatosis) and microscopic polyangiitis
For other autoimmune diseases, the effectiveness and safety of MabThera® have not been established.
Infusion reactions. The development of infusion reactions may be due to the release of cytokines and/or other mediators. Before each infusion of MabThera®, premedication with an analgesic/antipyretic and an antihistamine is necessary. In addition, before each infusion with MabThera®, patients with rheumatoid arthritis should receive premedication with GCS to reduce the frequency and severity of infusion reactions.
In most cases, infusion reactions in patients with rheumatoid arthritis were mild or moderate in severity. Severe infusion reactions with fatal outcomes have been reported during post-marketing experience. It is necessary to carefully monitor patients with previously identified diseases of the cardiovascular system, as well as those who have previously experienced adverse reactions from the heart and lungs. The most commonly observed infusion reactions were headache, itching, sore throat, hot flashes, rash, urticaria, increased blood pressure and fever. Infusion reactions were observed more often after the first infusion of any course of treatment than after the second infusion. Subsequent infusions of MabThera® were better tolerated than the first. Serious infusion reactions were observed in less than 1% of patients, most often during the first infusion of the first cycle. Infusion reactions disappear after slowing down or interrupting the administration of MabThera® and drug therapy (antipyretics, antihistamines and sometimes oxygen, intravenous administration of 0.9% sodium chloride solution, bronchodilators and, if necessary, corticosteroids). If infusion reactions develop, depending on their severity and the required treatment, the administration of MabThera should be temporarily suspended or discontinued.
In most cases, after complete resolution of symptoms, the infusion can be resumed at a rate of 50% of the previous rate (for example, 50 mg/hour instead of 100 mg/hour).
The infusion reactions observed in granulomatosis with polyangiitis and microscopic polyangiitis were consistent with those already described in rheumatoid arthritis. The lower frequency and severity of infusion reactions in granulomatosis with polyangiitis and microscopic polyangiitis could be associated with the use of high doses of corticosteroids.
Due to the potential for the development of anaphylactic reactions and other immediate hypersensitivity reactions with intravenous administration of protein drugs, it is necessary to have means to relieve them: adrenaline, antihistamines and corticosteroids.
Side effects from the cardiovascular system. Due to the possibility of arterial hypotension, antihypertensive medications should be discontinued at least 12 hours before MabThera infusion.
Careful monitoring of patients with a history of cardiovascular disease is required due to the possibility of developing angina or arrhythmia (atrial flutter and fibrillation), heart failure or myocardial infarction.
Infections. Due to a possible increased risk of infectious complications, MabThera should not be administered to patients with acute infection or severe immunodeficiency (hypogammaglobulinemia or low CD4, CD8 levels). Caution should be exercised when prescribing MabThera® in patients with chronic infection or in the presence of conditions predisposing to the development of serious infections. If an infectious complication occurs, appropriate therapy should be prescribed.
Hepatitis B. Cases of reactivation of the hepatitis B virus (including fatal outcomes) have been observed when using MabThera® in patients with rheumatoid arthritis, granulomatosis with polyangiitis and microscopic polyangiitis. Before prescribing MabThera®, all patients should be screened for hepatitis B. The minimum set of tests should include determination of HBsAg and HBcAb, in accordance with local recommendations, the list of tests can be expanded. MabThera® should not be used in patients with active hepatitis B. Patients with positive serological markers for hepatitis B should consult a hepatologist before using MabThera®; For such patients, appropriate monitoring and measures should be taken to prevent hepatitis B virus reactivation in accordance with local standards.
Progressive multifocal leukoencephalopathy (PML). During the period of post-registration use of MabThera® in patients with autoimmune diseases, incl. with rheumatoid arthritis, fatal cases of PML have been observed. Some patients had multiple risk factors for PML: comorbidities, long-term use of immunosuppressive therapy or chemotherapy. Cases of PML have also been reported in patients with autoimmune diseases not receiving MabThera®. If neurological symptoms occur in such patients, it is necessary to conduct a differential diagnosis to exclude PML and consult a neurologist.
Skin reactions. Cases of the development of such severe skin reactions as toxic epidermal necrolysis and Stevens-Johnson syndrome, in some cases with a fatal outcome, have been reported. If these reactions are detected, MabThera® should be discontinued.
Immunization. The safety and effectiveness of immunization with live viral vaccines following treatment with MabThera® has not been studied. Vaccination with live viral vaccines is not recommended. Vaccination with inactivated vaccines is possible, but response rates may be reduced.
Before using MabThera® in patients with rheumatoid arthritis, the patient’s vaccination status should be examined and appropriate recommendations should be followed. Vaccinations should be completed at least 4 weeks before rituximab administration.
After 6 months of therapy with MabThera® and methotrexate, a decrease in the response rate to the administration of polysaccharide pneumococcal vaccine (43% versus 82%, at least 2 serotypes of antibodies to pneumococcus), KHL neoantigen (KHL-hemocyanin fissurelia molluscum) (34% versus 80%) was observed compared with methotrexate monotherapy. After treatment with MabThera® and methotrexate, the response rate to tetanus toxoid was similar to that after methotrexate monotherapy (39% versus 42%).
If necessary, vaccination with inactivated vaccines should be completed at least 4 weeks before the second course of therapy.
The number of patients with rheumatoid arthritis and positive titers of antibodies to Streptococcus pneumoniae, influenza A, mumps, rubella, chickenpox and tetanus toxin before and 1 year after the start of MabThera therapy did not change.
Antichimeric antibodies. The appearance of antichimeric antibodies in most patients with rheumatoid arthritis is not accompanied by clinical manifestations or an increased risk of reactions during subsequent infusions, but rarely their presence may be associated with more severe allergic or infusion reactions with repeated infusions during subsequent courses and a lack of effect in reducing the B-cell pool during subsequent courses of therapy.
Patients with rheumatoid arthritis who have not previously received methotrexate. MabThera® is not recommended for the treatment of patients who have not previously received methotrexate, because a favorable benefit/risk ratio for this category of patients has not been confirmed.
Use in pediatrics
The safety and effectiveness of the drug in children have not been established. When using MabThera® in children, hypogammaglobulinemia was observed, in some cases in severe form, requiring long-term immunoglobulin replacement therapy. The consequences of B cell depletion in children are unknown. Disposal Disposal of MabThera® should be carried out in accordance with local requirements.
Impact on the ability to drive vehicles and other mechanisms that require increased concentration
Whether rituximab affects the ability to drive and operate machines is unknown, although the pharmacological activity and reported adverse events do not suggest such an effect.
Active ingredient
Active ingredient
Rituximab
Composition
Composition
1 ml (1 bottle) contains:
Active ingredients:
rituximab 10 mg (500 mg).
Excipients:
sodium citrate dihydrate – 7.35 mg,
polysorbate 80 – 0.7 mg,
sodium chloride – 9 mg,
hydrochloric acid or sodium hydroxide (up to pH 6.5),
water for d/i – up to 1 ml.
The bottle contains 50 ml of concentrate.
There is 1 bottle in a cardboard package.
Pregnancy
Pregnancy
Immunoglobulins G (IgG) are able to penetrate the placental barrier.
The level of B cells in newborns has not been studied when MabThera is prescribed to women during pregnancy.
Transient B cell depletion and lymphocytopenia have been observed in some neonates whose mothers received rituximab during pregnancy. Therefore, MabThera® should not be prescribed to pregnant women unless the potential benefits of therapy outweigh the potential risks.
During the treatment period and for 12 months after the end of treatment with MabThera®, women of childbearing age should use effective methods of contraception.
It is not known whether rituximab is excreted in breast milk. Considering that IgG class immunoglobulins circulating in the mother’s blood are excreted in breast milk, MabThera® should not be used during breastfeeding.
Contraindications
Contraindications
Hypersensitivity to rituximab, any component of the drug or mouse proteins;
acute infectious diseases;
severe primary or secondary immunodeficiency;
severe heart failure (NYHA class IV) with rheumatoid arthritis;
children and adolescents under 18 years of age (efficacy and safety have not been established);
pregnancy;
breastfeeding period.
With caution: use with a history of respiratory failure or tumor infiltration of the lungs; with the number of circulating malignant cells >25,000/μl or a high tumor load; neutropenia (less than 1500/μl), thrombocytopenia (less than 75,000/μl); for chronic infections.
Side Effects
Side Effects
The following criteria are used to assess the frequency of adverse reactions:
very often (≥10%);
often (≥1%-<10%);
uncommon (≥0.1%-<1%).
Experience in using the drug for oncohematological diseases
MabThera® for the treatment of low-grade or follicular non-Hodgkin’s lymphoma – monotherapy/maintenance therapy
Adverse reactions were reported up to 12 months after monotherapy and up to 1 month after MabThera maintenance therapy.
Infectious and parasitic diseases: very often – bacterial and viral infections; often – respiratory tract infections*, pneumonia*, sepsis, herpes zoster*, infections accompanied by fever*, fungal infections, infections of unknown etiology.
From the blood and lymphatic system: very often – leukopenia, neutropenia; often – thrombocytopenia, anemia; uncommon – lymphadenopathy, bleeding disorder, transient partial aplastic anemia, hemolytic anemia.
From the respiratory system, chest and mediastinal organs: often – rhinitis, bronchospasm, cough, respiratory diseases, shortness of breath, chest pain; uncommon – hypoxia, impaired pulmonary function, bronchiolitis obliterans, bronchial asthma.
From the immune system: very often – angioedema; often – hypersensitivity reactions.
Metabolism and nutrition: often – hyperglycemia, weight loss, peripheral edema, facial edema, increased LDH activity, hypocalcemia.
General disorders and disorders at the injection site: very often – headache, fever, chills, asthenia; often – pain in tumor foci, flu-like syndrome, hot flashes, weakness; infrequently – pain at the injection site.
From the gastrointestinal tract: very often – nausea; often – vomiting, diarrhea, dyspepsia, lack of appetite, dysphagia, stomatitis, constipation, abdominal pain, sore throat; infrequently – abdominal enlargement.
From the cardiovascular system: often – decreased blood pressure, increased blood pressure, orthostatic hypotension, tachycardia, arrhythmia, atrial fibrillation*, myocardial infarction*, cardiac pathology*; uncommon – left ventricular heart failure*, ventricular and supraventricular tachycardia*, bradycardia, myocardial ischemia*, angina*.
From the nervous system: often – dizziness, paresthesia, hypoesthesia, sleep disturbance, anxiety, agitation, vasodilation; infrequently – a perversion of taste.
From the mental side: infrequently – nervousness, depression.
From the musculoskeletal and connective tissue side: often – myalgia, arthralgia, muscle hypertonicity, back pain, neck pain, pain.
From the skin and subcutaneous tissues: very often – itching, rash; often – urticaria, increased sweating at night, sweating, alopecia*.
On the part of the organ of vision: often – impaired lacrimation, conjunctivitis.
From the organ of hearing and labyrinthine disorders: often – pain and tinnitus.
Laboratory and instrumental data: very often – a decrease in the concentration of immunoglobulins G (IgG). *frequencies are indicated only for adverse reactions of ≥3 severity in accordance with the National Cancer Institute toxicity criteria (NCI-CTC).
MabThera® in combination with chemotherapy (R-CHOP, R-CVP, R-FC) for non-Hodgkin’s lymphoma and chronic lymphocytic leukemia
The following are severe adverse reactions in addition to those observed with monotherapy/maintenance therapy and/or occurring at a higher frequency.
Infectious and parasitic diseases: very often – bronchitis; often – acute bronchitis, sinusitis, hepatitis B* (reactivation of the hepatitis B virus and primary infection).
From the blood and lymphatic system: very often – neutropenia**, febrile neutropenia, thrombocytopenia; often – pancytopenia, granulocytopenia.
From the skin and subcutaneous tissues: very often – alopecia; often – skin diseases.
General disorders and disorders at the injection site: often – fatigue, chills.
* – frequency is indicated based on observations during the treatment of recurrent/chemoresistant chronic lymphocytic leukemia according to the R-FC regimen.
** – prolonged and/or delayed neutropenia was observed after completion of therapy according to the R-FC regimen in previously untreated patients or in patients with relapsed/chemoresistant chronic lymphocytic leukemia.
The following are adverse reactions that occurred during therapy with MabThera® with the same frequency (or less often) compared to the control group: hematotoxicity, neutropenic infections, urinary tract infections, septic shock, lung superinfections, implant infections, staphylococcal septicemia, mucous discharge from the nose, pulmonary edema, heart failure, sensory disturbances, venous thrombosis, incl. deep vein thrombosis of the extremities, mucositis, edema of the lower extremities, decreased left ventricular ejection fraction, increased body temperature, deterioration of general health, fall, multiple organ failure, bacteremia, decompensation of diabetes mellitus.
The safety profile of MabThera in combination with chemotherapy regimens MCP, CHVP-IFN does not differ from that in combination with CVP, CHOP or FC in relevant populations.
Infusion reactions
Monotherapy with MabThera® (for 4 weeks)
More than 50% of patients experienced events resembling infusion reactions, most often during the first infusions. Infusion reactions include chills, trembling, weakness, shortness of breath, nausea, rash, hot flashes, low blood pressure, fever, itching, urticaria, irritation of the tongue or swelling of the larynx (angioedema), rhinitis, vomiting, pain in tumor areas, headache, bronchospasm. The development of signs of tumor lysis syndrome has been reported.
MabThera® in combination with chemotherapy according to the following regimens: R-CVP for non-Hodgkin’s lymphoma; R-CHOP for diffuse large B-cell non-Hodgkin lymphoma; R-FC for chronic lymphocytic leukemia
Grade 3 and 4 infusion reactions during infusion or within 24 hours after infusion of MabThera® were observed during the first cycle of chemotherapy in 12% of patients. The incidence of infusion reactions decreased with each subsequent cycle and by the 8th cycle of chemotherapy, the incidence of infusion reactions reached less than 1%. Infusion reactions, in addition to those mentioned above (with monotherapy with MabThera®), included: dyspepsia, rash, increased blood pressure, tachycardia, signs of tumor lysis syndrome, in some cases – myocardial infarction, atrial fibrillation, pulmonary edema and acute reversible thrombocytopenia.
Infections
Monotherapy with MabThera® (for 4 weeks)
MabThera® causes depletion of the B cell pool in 70-80% of patients and a decrease in serum immunoglobulin concentrations in a small number of patients. Bacterial, viral, fungal infections and infections of unspecified etiology (all, regardless of cause) develop in 30.3% of patients. Severe infections (grades 3 and 4), including sepsis, were noted in 3.9% of patients.
Maintenance therapy (non-Hodgkin’s lymphoma) up to 2 years
During therapy with MabThera®, an increase in the overall incidence of infections was observed, incl. infections of 3-4 severity. There was no increase in the incidence of infectious complications with maintenance therapy lasting 2 years. Fatal progressive multifocal leukoencephalopathy (PML) has been reported in patients with non-Hodgkin’s lymphoma after disease progression and re-treatment.
MabThera® in combination with chemotherapy according to the following regimens: R-CVP for non-Hodgkin’s lymphoma; R-CHOP for diffuse large B-cell non-Hodgkin lymphoma; R-FC for chronic lymphocytic leukemia
There was no increase in the incidence of infections or infestations with MabThera® R-CVP regimen. Upper respiratory tract infections were most common (12.3% in the R-CVP group). Serious infections occurred in 4.3% of patients receiving R-CVP chemotherapy; No life-threatening infections were reported.
The proportion of patients with grade 2-4 infections and/or febrile neutropenia in the R-CHOP group was 55.4%. The overall incidence of grade 2–4 infections in the R-CHOP group was 45.5%. The incidence of grade 2-4 fungal infections in the R-CHOP group was higher than in the CHOP group due to a higher incidence of local candidiasis and amounted to 4.5%. The incidence of grade 2-4 herpes infection was higher in the R-CHOP group compared to the CHOP group and was 4.5%.
In patients with chronic lymphocytic leukemia, the incidence of hepatitis B (hepatitis B virus reactivation and primary infection) of grade 3-4 in the R-FC group was 2%.
From the hematopoietic system
Monotherapy with MabThera® (for 4 weeks)
1.7% – severe thrombocytopenia (grade 3 and 4); 4.2% – severe neutropenia; 1.1% – severe anemia (grade 3 and 4).
Maintenance therapy (non-Hodgkin’s lymphoma) up to 2 years
Leukopenia (grades 3 and 4) was observed in 5% of patients, neutropenia (grades 3 and 4) in 10% of patients receiving MabThera®. The incidence of thrombocytopenia (grade 3-4) during therapy with MabThera was low and amounted to <1%.
Approximately 50% of patients for whom B-cell recovery data were available took 12 months or more to recover B-cell counts to normal levels after completion of induction therapy with MabThera.
MabThera® in combination with chemotherapy according to the following regimens: R-CVP for non-Hodgkin’s lymphoma; R-CHOP for diffuse large B-cell non-Hodgkin lymphoma; R-FC for chronic lymphocytic leukemia
Severe neutropenia and leukopenia (grades 3 and 4): in patients receiving MabThera® in combination with chemotherapy, leukopenia of grades 3 and 4 was observed more often compared to patients receiving chemotherapy alone. The incidence of severe leukopenia was 88% in patients receiving R-CHOP and 23% in patients receiving R-FC. The incidence of severe neutropenia was 24% in the R-CVP group, 97% in the R-CHOP group, and 30% in the R-FC group in previously untreated chronic lymphocytic leukemia. The higher incidence of neutropenia in patients receiving MabThera and chemotherapy was not associated with an increased incidence of infections and infestations compared with patients receiving chemotherapy alone. In patients with recurrent or chemoresistant chronic lymphocytic leukemia after treatment according to the R-FC regimen, in some cases neutropenia was characterized by a long course or later manifestations.
Severe anemia and thrombocytopenia (grades 3 and 4): there was no significant difference in the incidence of anemia of grades 3 and 4 between groups. In the R-FC group, in the first line of treatment for chronic lymphocytic leukemia, anemia of grades 3 and 4 occurred in 4% of patients, thrombocytopenia of grades 3 and 4 – in 7% of patients. In the R-FC group with recurrent or chemoresistant chronic lymphocytic leukemia, anemia of grades 3 and 4 occurred in 12% of patients, thrombocytopenia of grades 3 and 4 – in 11% of patients.
From the nervous system
MabThera® in combination with chemotherapy according to the following regimens: R-CVP for non-Hodgkin’s lymphoma; R-CHOP for diffuse large B-cell non-Hodgkin lymphoma; R-FC for chronic lymphocytic leukemia
Patients (2%) in the R-CHOP group with cardiovascular risk factors developed cerebroembolic events during the first cycle of therapy, in contrast to patients in the CHOP group who developed cerebroembolic events during the observation period without treatment. There was no difference between groups in the incidence of other thromboembolism.
The overall incidence of grade 3 and 4 neurological impairment was low both in the first-line treatment of chronic lymphocytic leukemia (4% in the R-FC group) and in the treatment of relapsed/chemoresistant chronic lymphocytic leukemia (3% in the R-FC group).
IgG concentration
Maintenance therapy (non-Hodgkin’s lymphoma) up to 2 years
After induction therapy, IgG concentrations were below the lower limit of normal (<7 g/L) in the MabThera®-treated group and in the untreated group. In the group not receiving MabThera®, the median IgG concentration increased consistently and exceeded the lower limit of normal, while the median IgG concentration did not change in the group receiving MabThera®. In 60% of patients treated with MabThera® for 2 years, IgG concentrations remained below the lower limit. In the group without MabThera® therapy, after 2 years, IgG concentrations remained below the lower limit in 36% of patients.
Special categories of patients
Monotherapy with MabThera® (for 4 weeks)
In elderly patients (65 years and older), the frequency and severity of all grade 3 and 4 adverse reactions and side effects do not differ from those in younger patients.
Combination therapy
In elderly patients (65 years and older) during first-line therapy, as well as during treatment of relapsed/chemoresistant chronic lymphocytic leukemia, the incidence of grade 3 and 4 adverse reactions from the blood and lymphatic system was higher compared to younger patients.
With a high tumor load (the diameter of single lesions is more than 10 cm), the frequency of adverse reactions of grade 3 and 4 is increased.
With repeated therapy, the frequency and severity of adverse reactions do not differ from those during initial therapy.
Experience in using the drug for rheumatoid arthritis
Below are the adverse reactions that occurred during treatment with MabThera® with a frequency of at least 2% and with at least a 2% difference compared to the control group.
On the part of the immune system, general disorders and disorders at the injection site: very often – infusion reactions * (often – increased and decreased blood pressure, hot flashes, rash, urticaria, itching, chills, fever, nausea, rhinitis, sore throat, tachycardia, weakness, pain in the mouth and pharynx, peripheral edema, erythema).
* – the following clinically significant infusion reactions were also rarely observed: generalized edema, bronchospasm, wheezing, laryngeal edema, angioedema, generalized itching, anaphylaxis, anaphylactoid reaction. Infectious and parasitic diseases: very often – urinary tract infections, upper respiratory tract infections; often – bronchitis, sinusitis, gastroenteritis, dermatophytosis of the feet.
From the digestive system: often – dyspepsia, diarrhea, gastroesophageal reflux, ulceration of the oral mucosa, pain in the right upper quadrant of the abdomen.
From the nervous system: very often – headache; often – migraine, paresthesia, dizziness, sciatica.
Mental disorders: often – depression, anxiety.
From the musculoskeletal system and connective tissue: often – arthralgia, musculoskeletal pain, osteoarthritis, bursitis.
From the skin and subcutaneous tissues: often – alopecia.
Laboratory and instrumental data: often – hypercholesterolemia.
Repeated therapy: the profile of adverse reactions during repeated use does not differ from that during initial therapy. The safety profile improved with each subsequent course of therapy and was characterized by a decrease in the incidence of infusion reactions, infections and exacerbations of the disease, which were most common in the first 6 months of therapy.
Infusion reactions: Infusion reactions were the most frequently reported adverse reaction with MabThera. 35% of patients experienced at least one infusion reaction, with serious infusion reactions occurring in less than 1% of patients, regardless of dose. In most cases, infusion reactions were grade 1 and 2. The proportion of grade 3 infusion reactions and infusion reactions leading to discontinuation of therapy decreased with each subsequent course of treatment, and, starting from course 3, such reactions were observed rarely. There were no grade 4 infusion reactions or deaths due to their development.
In 23% of patients, after the first administration of MabThera, the following symptoms of infusion reactions occurred: nausea, itching, fever, urticaria/rash, chills, trembling, sneezing, angioedema, pharyngeal irritation, cough and bronchospasm with or without an increase or decrease in blood pressure. Premedication with intravenous corticosteroids significantly reduces the frequency and severity of such events.
Infections: During treatment with MabThera, the overall incidence of infections, which were predominantly mild to moderate in severity (most commonly upper respiratory tract infections and urinary tract infections), was 97 per 100 patient-years. The incidence of severe infections, some of which were fatal, was 4 per 100 patient-years. Clinically significant serious adverse events also included pneumonia (1.9%).
Malignant diseases: the incidence of malignant diseases after the use of MabThera® does not exceed the rates in the age- and sex-matched population and is 0.8 per 100 patient-years.
From laboratory parameters: hypogammaglobulinemia (decrease in the concentration of immunoglobulins IgG and IgM below the lower limit of normal), not accompanied by an increase in the overall frequency of infections or the frequency of serious infections. During the first course of therapy with MabThera®, incl. several months after completion of therapy, cases of neutropenia, mostly transient and mild to moderate in severity, have been reported. At the same time, the frequency of severe neutropenia (grades 3 and 4) was 0.94% compared to 0.27% in the group that did not receive the drug.
Considering that after the first course of therapy with MabThera, the incidence of severe neutropenia was 1.06 per 100 patient-years compared to 0.53 per 100 patient-years without such therapy, and after repeated use, the incidence of severe neutropenia was 0.97 per 100 patient-years compared to 0.88 per 100 patient-years without such therapy, severe neutropenia can be considered an adverse reaction only in relation to first course of therapy with MabThera®. The time of manifestation of neutropenia varied. Neutropenia was not associated with an increase in the incidence of serious infections, and in most cases, patients received repeat courses of MabThera after episodes of neutropenia.
Experience in using the drug for granulomatosis with polyangiitis (Wegener’s granulomatosis) and microscopic polyangiitis
The following are adverse events that were observed with MabThera® at an incidence of ≥10% (very common) compared with the incidence of adverse reactions with cyclophosphamide (crossover or substitution with another therapy was allowed based on informed clinical judgment).
Infectious and parasitic diseases: infections, including the most common infections of the upper respiratory tract, urinary tract infections, herpes zoster – 61.6% (in the comparison group – 46.9%).
From the gastrointestinal tract: nausea – 18.2% (in the comparison group – 20.4%), diarrhea – 17.2% (in the comparison group – 12.2%).
From the nervous system: headache – 17.2% (in the comparison group -19.4%).
From the musculoskeletal system and connective tissue: muscle spasms – 17.2% (in the comparison group – 15.3%), arthralgia – 13.1% (in the comparison group – 9.2%).
From the blood and lymphatic system: anemia – 16.2% (in the comparison group – 20.4%), leukopenia – 10.1% (in the comparison group – 26.5%).
General disorders and disorders at the injection site: peripheral edema – 16.2% (in the comparison group – 6.1%), weakness – 13.1% (in the comparison group – 21.4%).
From the immune system: infusion reactions, including the most common, cytokine release syndrome, redness, throat irritation, tremor – 12.1% (in the comparison group – 11.2%).
Mental disorders: insomnia – 14.1% (in the comparison group – 12.2%).
Laboratory and instrumental data: increased ALT activity – 13.1% (in the comparison group – 15.3%).
From the respiratory system, chest organs and mediastinum: cough – 13.1% (in the comparison group – 11.2%), nosebleeds 11.1% (in the comparison group – 6.1%), dyspnea – 10.1% (in the comparison group – 11.2%).
From the cardiovascular system: increased blood pressure – 12.1% (in the comparison group – 5.1%).
From the skin and subcutaneous tissues: rash – 10.1% (in the comparison group – 17.3%).
Infusion reactions: all infusion reactions observed during the infusion of MabThera® or within 24 hours after it were grade 1 and 2. The most commonly observed symptoms were cytokine release syndrome, flushing, throat irritation, and tremors. The use of MabThera® in combination with intravenous corticosteroids could reduce the frequency and severity of the described adverse reactions.
Infections: The overall incidence of infections with MabThera was 210 per 100 patient-years. Infections were predominantly mild to moderate and most commonly included upper respiratory tract infections, urinary tract infections, and herpes zoster. The incidence of serious infections with MabThera was 25 per 100 patient-years. Among serious infections with MabThera® use, the most commonly reported infection was pneumonia (4%).
Malignant diseases: the incidence of new cases of malignant diseases with the use of MabThera® corresponds to the rates in the population and is 2.05 per 100 patient-years.
From the laboratory parameters: hypogammaglobulinemia (decrease in the concentration of immunoglobulins below the lower limit of normal) IgA, IgG and IgM at 6 months of therapy in the MabThera® group was 27%, 58% and 51%, respectively, compared with 25%, 50% and 46% in the comparison group. In patients with low concentrations of IgA, IgG, and IgM, there was no increase in the overall incidence of infections or the incidence of serious infections.
Neutropenia grades 3 and 4 was observed in 24% of patients in the MabThera group and in 23% of patients in the comparison group. There was no increase in the incidence of serious infections associated with neutropenia in patients receiving rituximab. The effect of rituximab on the development of neuropenia with repeated use has not been studied.
Post-registration use of MabThera® in non-Hodgkin’s lymphoma and chronic lymphocytic leukemia
From the cardiovascular system: severe cardiovascular events associated with infusion reactions, such as heart failure and myocardial infarction, mainly in patients with a history of cardiovascular disease and/or receiving cytotoxic chemotherapy; very rarely – vasculitis, mainly cutaneous (leukocytoclastic).
From the respiratory system: respiratory failure and pulmonary infiltrates caused by infusion reactions; In addition to pulmonary adverse reactions due to infusion reactions, interstitial lung disease, in some cases fatal, has been observed.
From the circulatory and lymphatic system: reversible acute thrombocytopenia associated with infusion reactions.
From the skin and its appendages: rarely – severe bullous reactions, including toxic epidermal necrolysis and Stevens-Johnson syndrome, in some cases fatal.
From the nervous system: rarely – neuropathy of the cranial nerves in combination with peripheral neuropathy or without it (marked decrease in visual acuity, hearing, damage to other sensory organs, paresis of the facial nerve) during various periods of therapy up to several months after completion of the course of treatment with MabThera®. Posterior reversible encephalopathy syndrome (PRES)/posterior reversible leukoencephalopathy syndrome (PRLS) have been reported in patients treated with MabThera. Symptoms included blurred vision, headache, seizures and mental disturbances, with or without increased blood pressure. The diagnosis of PRES/PRLS can be confirmed using brain imaging techniques. In the described cases, patients had risk factors for the development of PRES/PRLS, such as underlying disease, elevated blood pressure, immunosuppressive therapy and/or chemotherapy.
From the body as a whole and reactions at the injection site: rarely – serum sickness.
Infections: reactivation of viral hepatitis B (in most cases with a combination of MabThera® and cytotoxic chemotherapy); as well as other severe viral infections (primary infection, viral reactivation or exacerbation), some of which were accompanied by death, caused by cytomegalovirus, Varicella zoster, Herpes simplex, JC polyomavirus (PML), hepatitis C virus. When MabThera® was prescribed for indications not covered by the instructions for medical use, sarcoma progression was observed in patients with previously diagnosed Kaposi’s sarcoma (most patients were HIV positive).
From the gastrointestinal tract: perforation of the stomach and/or intestines (possibly fatal) when MabThera® is combined with chemotherapy for non-Hodgkin’s lymphoma.
From the blood and lymphatic system: rarely – neutropenia that occurred 4 weeks after the last administration of rituximab; a transient increase in IgM concentration in patients with Waldenström’s macroglobulinemia, followed by a return to its original value after 4 months.
Post-registration use of MabThera® for rheumatoid arthritis, granulomatosis with polyangiitis (Wegener’s granulomatosis) and microscopic polyangiitis
The following are adverse reactions that were observed in patients with rheumatoid arthritis during post-marketing use of MabThera®, and are also expected or observed in patients with granulomatosis with polyangiitis (Wegener’s granulomatosis) and microscopic polyangiitis.
Infections: PML, hepatitis B virus reactivation.
On the part of the body as a whole, reactions at the injection site: reactions resembling serum sickness; severe infusion reactions, in some cases fatal.
From the skin and its appendages: very rarely – toxic epidermal necrolysis and Stevens-Johnson syndrome, in some cases with a fatal outcome.
From the blood and lymphatic system: rarely – neutropenia (including severe cases with late manifestation and cases of prolonged neutropenia), some of which have been associated with fatal infections.
From the nervous system: cases of PRES/PRLS have been observed in patients receiving MabThera®. Symptoms included blurred vision, headache, seizures and mental disorders, with or without increased blood pressure. The diagnosis of PRES/PRLS can be confirmed using brain imaging techniques. In the described cases, patients had risk factors for the development of PRES/PRLS, such as elevated blood pressure, immunosuppressive therapy and/or other concomitant therapy.
Interaction
Interaction
Data on drug interactions with MabThera® are limited.
In patients with chronic lymphocytic leukemia, with simultaneous use of MabThera®, fludarabine and cyclophosphamide, the pharmacokinetic parameters do not change.
Concomitant use of methotrexate does not affect the pharmacokinetics of rituximab in patients with rheumatoid arthritis.
When prescribed with other monoclonal antibodies for diagnostic or therapeutic purposes to patients who have antibodies against mouse proteins or anti-chimeric antibodies, the risk of allergic reactions increases.
In patients with rheumatoid arthritis, the incidence of serious infections during therapy with MabThera (before therapy with other biologic disease-modifying anti-inflammatory drugs (DMARDs)) is 6.1 per 100 patient-years, while during subsequent therapy with other DMARDs it is 4.9 per 100 patient-years.
When administering MabThera®, polyvinyl chloride or polyethylene infusion systems or bags may be used due to the compatibility of the material with the drug.
Overdose
Overdose
Cases of overdose in humans have not been observed.
Single doses of rituximab greater than 1000 mg have not been studied.
The maximum dose of 5000 mg was prescribed to patients with chronic lymphocytic leukemia; no additional safety data were obtained.
Due to the increased risk of infectious complications when the B-lymphocyte pool is depleted, the infusion of MabThera® should be discontinued, the patient’s condition should be monitored, and a complete blood count should be ordered.
Storage conditions
Storage conditions
In a place protected from light, at a temperature of 2–8 °C
Shelf life
Shelf life
2.5 years
Manufacturer
Manufacturer
Roche Diagnostics GmbH, Germany
Additional information
| Shelf life | 2.5 years |
|---|---|
| Conditions of storage | In a light-protected place, at 2-8 °C |
| Manufacturer | Roche Diagnostics GmbH, Germany |
| Medication form | concentrate for preparation of infusion solution |
| Brand | Roche Diagnostics GmbH |
Related products
Buy Mabthera, 500 mg/50 ml 50 ml with delivery to USA, UK, Europe and over 120 other countries.