No products in the cart.
Lozap, 50 mg 90 pcs.
€17.35 €15.03
Description
Lozap is a hypotensive.
Pharmacodynamics
The hypotensive drug, is a specific angiotensin II receptor antagonist (AT1 subtype). Does not inhibit kinase II, an enzyme that degrades bradykinin. Reduces RPS, blood concentrations of adrenaline and aldosterone, BP, pressure in the small circulatory circle; reduces post-load, has a diuretic effect. Prevents the development of myocardial hypertrophy, increases exercise tolerance in patients with heart failure.
After a single dose, the hypotensive effect (decreases cAH and dAH) reaches a maximum after 6 hours, then gradually decreases over 24 hours.
The maximum hypotensive effect is reached 3-6 weeks after initiation of the drug.
Pharmacological data indicate that plasma concentrations of losartan are significantly increased in patients with cirrhosis, so patients with a history of liver disease should be prescribed at a lower dose.
Pharmacokinetics
Lozartan is rapidly absorbed from the GI tract. Bioavailability is about 33%. It has “first pass” effect through liver, metabolized by carboxylation with participation of CYP2C9 isoenzyme of cytochrome P450 with formation of active metabolite. Binding to plasma proteins is 99%. Time to reach Cmax losartan – 1 hour, active metabolite – 3-4 hours after oral administration. T1/2 is 1.5-2 h and its main metabolite is 6-9 h, respectively. About 35% of the dose is excreted with the urine, about 60% – through the intestine.
Indications
Indications
Arterial hypertension; heart failure (as part of combination therapy, with intolerance or ineffectiveness of therapy with ACE inhibitors).
Pharmacological effect
Pharmacological effect
Lozap – hypotensive.
Pharmacodynamics
The antihypertensive drug is a specific antagonist of angiotensin II receptors (AT1 subtype). Does not inhibit kinase II, an enzyme that destroys bradykinin. Reduces peripheral vascular resistance, blood concentrations of adrenaline and aldosterone, blood pressure, pressure in the pulmonary circulation; reduces afterload and has a diuretic effect. Prevents the development of myocardial hypertrophy, increases exercise tolerance in patients with heart failure.
After a single dose, the hypotensive effect (decreases SBP and DBP) reaches a maximum after 6 hours, then gradually decreases over 24 hours.
The maximum hypotensive effect is achieved 3–6 weeks after starting the drug.
Pharmacological data indicate that the plasma concentration of losartan in patients with liver cirrhosis is significantly increased, so patients with a history of liver disease should be prescribed a lower dose.
Pharmacokinetics
Losartan is rapidly absorbed from the gastrointestinal tract. Bioavailability – about 33%. It has a “first pass” effect through the liver, is metabolized by carboxylation with the participation of the CYP2C9 isoenzyme of cytochrome P450 to form an active metabolite. Plasma protein binding – 99%. The time to reach Cmax of losartan is 1 hour, the active metabolite is 3-4 hours after oral administration. T1/2 is 1.5–2 hours, and its main metabolite is 6–9 hours, respectively. About 35% of the dose is excreted in the urine, about 60% through the intestines.
Special instructions
Special instructions
It is necessary to correct dehydration before prescribing Lozap or begin treatment with the use of the drug at a lower dose.
Drugs that affect the renin-angiotensin system may increase blood urea and serum creatinine concentrations in patients with bilateral renal stenosis or arterial stenosis of a solitary kidney.
During the treatment period, the concentration of potassium in the blood should be regularly monitored, especially in elderly patients with impaired renal function.
Active ingredient
Active ingredient
Losartan
Composition
Composition
Active ingredient:
losartan potassium 50 mg;
Excipients:
MCC;
mannitol;
croscarmellose sodium;
povidone 30;
magnesium stearate;
hypromellose;
titanium dioxide;
talc;
propylene glycol
Contraindications
Contraindications
hypersensitivity to the components of the drug;
arterial hypotension;
hyperkalemia;
dehydration;
pregnancy;
lactation period;
age under 18 years (efficacy and safety have not been established).
Side Effects
Side Effects
From the nervous system and sensory organs: 1% or more – dizziness, asthenia, fatigue, headache, insomnia; less than 1% – anxiety, sleep disturbance, drowsiness, memory disorder, peripheral neuropathy, paresthesia, hypoesthesia, migraine, tremor, ataxia, depression, syncope, tinnitus, taste disturbance, vision changes, conjunctivitis.
From the respiratory system: 1% or more – nasal congestion, cough*, upper respiratory tract infections (fever, sore throat, sinusopathy*, sinusitis, pharyngitis); less than 1% – dyspnea, bronchitis, rhinitis.
From the gastrointestinal tract: 1% or more – nausea, diarrhea*, dyspeptic symptoms*, abdominal pain; less than 1% – anorexia, dry mouth, toothache, vomiting, flatulence, gastritis, constipation.
From the musculoskeletal system: 1% or more – convulsions, myalgia*, pain in the back, chest, legs; less than 1% – arthralgia, pain in the shoulder, knee, arthritis, fibromyalgia.
From the cardiovascular system: orthostatic hypotension (dose-dependent), palpitations, tachycardia or bradycardia, arrhythmia, angina pectoris, anemia.
From the genitourinary system: less than 1% – imperative urge to urinate, urinary tract infections, impaired renal function, weakened libido, impotence.
From the skin: less than 1% – dry skin, erythema, flushing, photosensitivity, increased sweating, alopecia.
Allergic reactions: less than 1% – urticaria, rash, itching, angioedema, including the face, lips, pharynx and/or tongue.
Other: hyperkalemia (serum potassium >5.5 mmol/l).
Interaction
Interaction
May be prescribed with other antihypertensive drugs.
There were no clinically significant interactions with hydrochlorothiazide, digoxin, indirect anticoagulants, cimetidine, or phenobarbital.
In patients with dehydration (previous treatment with large doses of diuretics), a pronounced decrease in blood pressure may occur.
Strengthens (mutually) the effect of other antihypertensive drugs (diuretics, beta-blockers, sympatholytics).
Increases the risk of hyperkalemia when used together with potassium-sparing diuretics and potassium supplements.
Overdose
Overdose
Symptoms: marked decrease in blood pressure, tachycardia; bradycardia may appear due to parasympathetic (vagal) stimulation.
Treatment: forced diuresis, symptomatic therapy; hemodialysis is not effective.
Storage conditions
Storage conditions
In a dry place, at a temperature not exceeding 30 °C
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
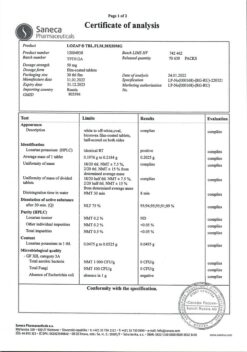
Saneka Pharmaceuticals a.s., Slovakia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a dry place, at a temperature no higher than 30 °C |
| Manufacturer | Saneka Pharmaceuticals a.s., Slovakia |
| Medication form | pills |
| Brand | Saneka Pharmaceuticals a.s. |
Other forms…
Related products
Buy Lozap, 50 mg 90 pcs. with delivery to USA, UK, Europe and over 120 other countries.