No products in the cart.
Lisinopril-Vertex, tablets 20 mg 30 pcs
€6.24 €5.20
Description
Pharmacodynamics
A ACE inhibitor, reduces the formation of angiotensin II from angiotensin I. Decreased angiotensin II leads to a direct reduction in aldosterone release. Reduces bradykinin degradation and increases prostaglandin synthesis. Reduces total peripheral vascular resistance (TPR), blood pressure (BP), preload, pulmonary capillary pressure, causes an increase in minute blood volume (MOB) and increases myocardial exercise tolerance in patients with chronic heart failure (CHF). Dilates arteries more than veins. Some effects are attributed to effects on the tissue renin-angiotensin-aldosterone system (RAAS). Long-term use reduces myocardial and arterial wall resistance hypertrophy. It improves the blood supply to the ischemic myocardium.
ACE inhibitors prolong life expectancy in patients with CHF and slow the progression of left ventricular (LV) dysfunction in patients who have had myocardial infarction without clinical manifestations of heart failure (CHF).
The onset of antihypertensive action is in 1 hour. The maximum effect is observed after 6-7 hours. Antihypertensive effect lasts for 24 hours. The duration of the effect also depends on the size of the dose. In arterial hypertension the effect is noted in the first days after the start of treatment, the stable effect develops after 1-2 months.
Pharmacokinetics
The average absorption of lisinopril is about 25%, with considerable interindividual variability (6-60%). Food does not affect the absorption of lisinopril.
Maximum plasma concentration of about 90 ng/ml is reached after 6 hours. Bioavailability is 25%. Weakly bound to plasma proteins. Blood-brain barrier and placental barrier permeability is low. It is practically not metabolized and is excreted unchanged by the kidneys. The elimination half-life is 12 hours. Absorption and clearance of lisinopril are decreased in patients with CHF, with cirrhosis and in elderly patients.
Disordered renal function leads to increased AUC (area under the curve “plasma concentration – time”) and elimination half-life of lisinopril, but these changes become clinically significant only when glomerular filtration rate is less than 30 ml/min.
Pharmacokinetics in selected patient groups
In patients with chronic heart failure, the absorption and clearance of lisinopril are reduced, with a bioavailability of 16%.
In patients with renal insufficiency (creatinine clearance less than 30 ml/min) the concentration of lisinopril is several times higher than in plasma of healthy volunteers with prolongation of time to maximum concentration in plasma and longer half-life.
In elderly patients the plasma concentration of lisinopril is increased by an average of 60% and the area under the curve “concentration-time” is 2 times greater than in younger patients.
In patients with cirrhosis, the bioavailability of lisinopril is reduced by 30% and clearance is reduced by 50% compared to patients with normal liver function.
Indications
Indications
– essential and renovascular arterial hypertension (AH) (in monotherapy or in combination with other antihypertensive drugs);
– chronic heart failure (CHF) (as part of combination therapy);
– early treatment of acute myocardial infarction as part of combination therapy (in the first 24 hours with stable hemodynamic parameters to maintain these parameters and prevent left ventricular dysfunction and heart failure);
– diabetic nephropathy (decreased albuminuria in patients with type 1 diabetes mellitus with normal blood pressure and patients with type 2 diabetes mellitus with arterial hypertension).
Pharmacological effect
Pharmacological effect
Pharmacodynamics
An ACE inhibitor, reduces the formation of angiotensin II from angiotensin I. A decrease in the content of angiotensin II leads to a direct decrease in the release of aldosterone. Reduces the degradation of bradykinin and increases the synthesis of prostaglandins. Reduces total peripheral vascular resistance (TPVR), blood pressure (BP), preload, pressure in the pulmonary capillaries, causes an increase in minute blood volume (MBV) and an increase in myocardial tolerance to stress in patients with chronic heart failure (CHF). Dilates arteries more than veins. Some effects are explained by effects on the tissue renin-angiotensin-aldosterone system (RAAS). With long-term use, hypertrophy of the myocardium and the walls of resistive arteries decreases. Improves blood supply to ischemic myocardium.
ACE inhibitors extend life expectancy in patients with CHF and slow down the progression of left ventricular (LV) dysfunction in patients who have suffered a myocardial infarction without clinical manifestations of heart failure (HF).
The onset of antihypertensive action is after 1 hour. The maximum effect is observed after 6-7 hours. The antihypertensive effect lasts for 24 hours. The duration of the effect also depends on the dose. In case of arterial hypertension, the effect is observed in the first days after the start of treatment, a stable effect develops after 1-2 months.
Pharmacokinetics
The average degree of absorption of lisinopril is about 25%, with significant interindividual variability (6-60%). Food does not affect the absorption of lisinopril.
The maximum plasma concentration of about 90 ng/ml is achieved after 6 hours. Bioavailability – 25%. Weakly binds to plasma proteins. Permeability through the blood-brain barrier and placental barrier is low. It is practically not metabolized and is excreted unchanged by the kidneys. The half-life is 12 hours. In patients with CHF, liver cirrhosis, and elderly patients, the absorption and clearance of lisinopril is reduced.
Impaired renal function leads to an increase in the AUC (area under the plasma concentration-time curve) and half-life of lisinopril, but these changes only become clinically significant when the glomerular filtration rate is less than 30 ml/min.
Pharmacokinetics in selected patient groups
In patients with chronic heart failure, absorption and clearance of lisinopril are reduced, bioavailability is 16%.
In patients with renal failure (creatinine clearance less than 30 ml/min), the concentration of lisinopril is several times higher than the concentration in the blood plasma of healthy volunteers, and there is an increase in the time to reach the maximum concentration in the blood plasma and an increase in the half-life.
In elderly patients, the concentration of lisinopril in the blood plasma is increased by an average of 60% and the area under the concentration-time curve is 2 times greater than in young patients.
In patients with liver cirrhosis, the bioavailability of lisinopril is reduced by 30% and clearance is reduced by 50% compared to patients with normal liver function.
Special instructions
Special instructions
A pronounced decrease in blood pressure most often occurs with a decrease in circulating blood volume caused by diuretic therapy, reducing the amount of salt in food, hemodialysis, diarrhea or vomiting. In patients with chronic heart failure with or without simultaneous renal failure, a pronounced decrease in blood pressure is possible. It is more often detected in patients with severe chronic heart failure, as a result of the use of large doses of diuretics, hyponatremia or impaired renal function. In such patients, treatment should be started under the strict supervision of a physician (with caution in selecting the dose of the drug and diuretics). Similar rules must be followed when prescribing to patients with coronary heart disease or cerebrovascular insufficiency, in whom a sharp decrease in blood pressure can lead to myocardial infarction or stroke. Before starting treatment, if possible, the sodium concentration should be normalized and/or the circulating blood volume should be replenished, and the effect of the initial dose of the drug on the patient should be carefully monitored. Treatment of symptomatic hypotension consists of bed rest and, if necessary, IV fluids (saline infusion). Transient arterial hypotension is not a contraindication for treatment with Lisinopril; however, it may require temporary discontinuation or dose reduction.
Treatment with Lisinopril is contraindicated in cases of cardiogenic shock and acute myocardial infarction, if the administration of a vasodilator can significantly worsen hemodynamic parameters, for example, when systolic blood pressure does not exceed 100 mmHg. Art.
In patients with acute myocardial infarction, decreased renal function (plasma creatinine concentration more than 177 µmol/l and/or proteinuria more than 500 mg/24 hours) is a contraindication for the use of Lisinopril. If renal failure develops during treatment with lisinopril (plasma creatinine concentration is more than 265 µmol/l or twice the initial level), the doctor must decide whether to discontinue treatment.
With bilateral renal artery stenosis and renal artery stenosis of a single kidney, as well as with hyponatremia and/or a decrease in circulating blood volume or circulatory failure, arterial hypotension caused by taking the drug Lisinopril can lead to a decrease in renal function with the subsequent development of reversible (after discontinuation of the drug) acute renal failure. A slight temporary increase in blood urea and creatinine concentrations may be observed in cases of impaired renal function, especially during concurrent treatment with diuretics. In cases of significant decrease in renal function (creatinine clearance less than 30 ml/min), caution and monitoring of renal function are required.
Angioedema of the face, extremities, lips, tongue, epiglottis and/or larynx has been reported rarely in patients treated with ACE inhibitors, including lisinopril, and may occur during any period of treatment. In this case, treatment with Lisinopril should be stopped as soon as possible and the patient should be monitored until complete regression of symptoms. In cases where swelling of only the face and lips occurs, the condition most often goes away without treatment, however, it is possible to prescribe antihistamines. Angioedema with laryngeal edema can be fatal. When the tongue, epiglottis or larynx are involved, airway obstruction may occur, so appropriate therapy (0.3-0.5 ml of epinephrine (adrenaline) solution 1:1000 subcutaneously, administration of glucocorticosteroids, antihistamines) and/or measures to ensure airway patency must be immediately carried out.
Patients who have a history of angioedema not related to previous treatment with ACE inhibitors may be at increased risk of developing it during treatment with an ACE inhibitor.
An anaphylactic reaction has also been observed in patients undergoing hemodialysis using high-flow dialysis membranes (AN69®) who are also taking lisinopril. In such cases, the use of a different type of dialysis membrane or another antihypertensive agent should be considered. Anaphylactic reactions may occur during apheresis of low-density lipoproteins with dextran sulfate.
In some cases of desensitization to the venom of Hymenoptera (wasps, bees, etc.), treatment with ACE inhibitors was accompanied by hypersensitivity reactions. This can be avoided if you first temporarily stop taking ACE inhibitors.
In patients undergoing major surgery or during general anesthesia, ACE inhibitors (in particular, lisinopril) can block the formation of angiotensin II. The decrease in blood pressure associated with this mechanism of action is corrected by an increase in circulating blood volume. Before surgery (including dentistry), you must warn the surgeon/anesthesiologist about the use of the drug Lisinopril.
The use of recommended doses of the drug in elderly patients may be accompanied by an increase in the concentration of lisinopril in the blood, so dose selection requires special attention and is carried out depending on the patient’s kidney function and blood pressure.
However, in elderly and young patients the antihypertensive effect of the drug Lisinopril is expressed to the same extent.
Cough has been reported when using ACE inhibitors. The cough is dry and prolonged, which disappears after stopping treatment with ACE inhibitors. When making a differential diagnosis of cough, cough caused by the use of ACE inhibitors must also be taken into account.
In some cases, hyperkalemia was observed. Risk factors for the development of hyperkalemia include renal failure, diabetes mellitus, and taking potassium supplements or drugs that increase potassium levels in the blood (eg, heparin), especially in patients with impaired renal function. During treatment with the drug, regular monitoring of blood plasma potassium ions, glucose, urea, and lipids is necessary.
During the treatment period, it is not recommended to drink alcoholic beverages, as alcohol enhances the antihypertensive effect of the drug.
Caution should be exercised when performing physical exercises and hot weather (risk of dehydration and excessive reduction in blood pressure due to a decrease in circulating blood volume).
Since the potential risk of agranulocytosis cannot be excluded, periodic monitoring of the blood picture is required.
Based on the results of epidemiological studies, it is assumed that the simultaneous use of ACE inhibitors and insulin, as well as oral hypoglycemic agents, can lead to the development of hypoglycemia. The greatest risk of development is observed during the first weeks of combination therapy, as well as in patients with impaired renal function. In patients with diabetes mellitus, careful glycemic control is required, especially during the first month of ACE inhibitor therapy. ACE inhibitors can lead to the development of cholestatic jaundice with progression to fulminant liver necrosis, so it is necessary to stop taking the drug if the activity of “liver” transaminases increases and symptoms of cholestasis appear.
Effect of the drug on the ability to drive vehicles and machinery
If side effects from the central nervous system occur, it is not recommended to drive vehicles, as well as perform work associated with increased risk.
Active ingredient
Active ingredient
Lisinopril
Composition
Composition
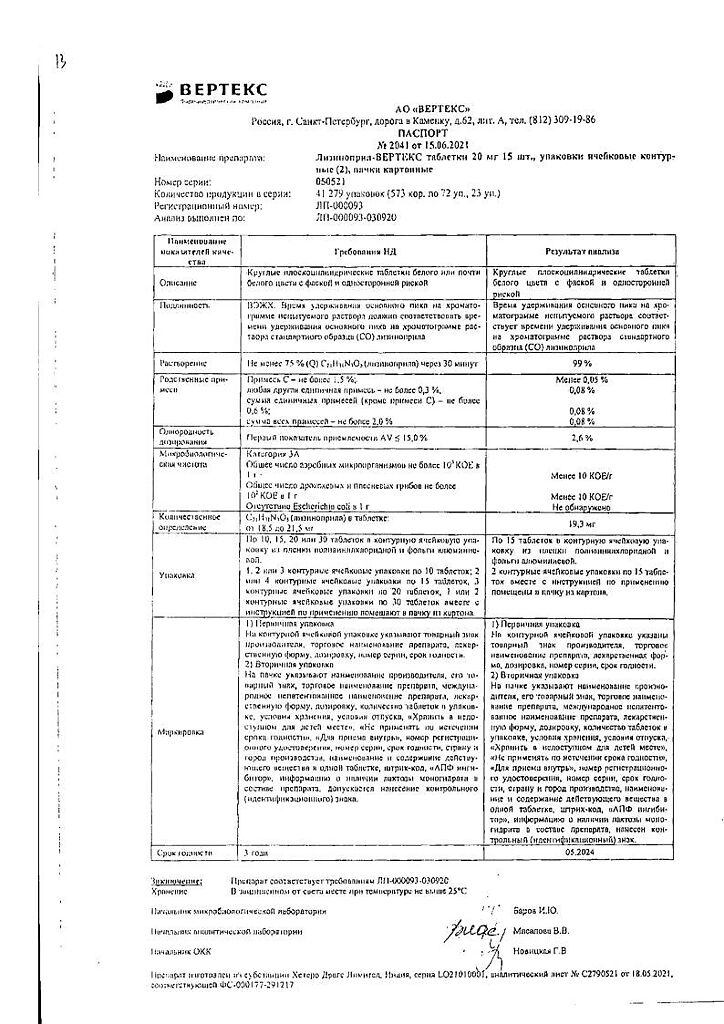
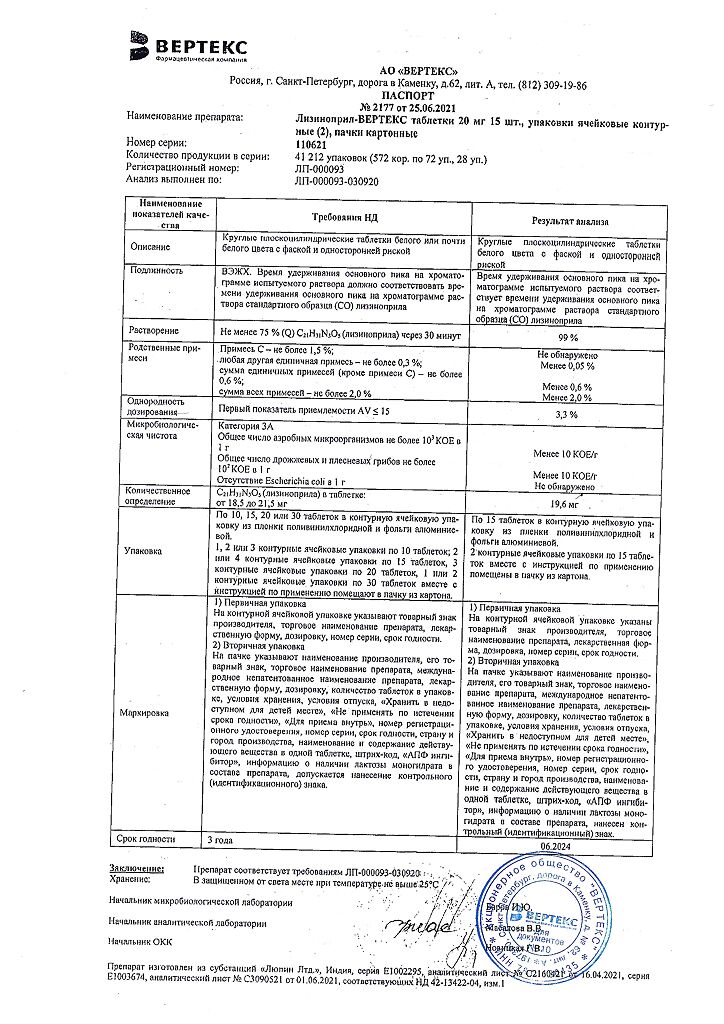
Each tablet contains:
Active ingredient:
lisinopril dihydrate (equivalent to lisinopril) 2.5 mg, 5 mg, 10 mg or 20 mg;
Excipients:
lactose monohydrate 77.5 mg/75.0 mg/70.0 mg/65.0 mg, respectively,
corn starch 49.0 mg,
povidone 6.0 mg,
talc 3.13 mg,
magnesium stearate 1.3 mg,
dyes (sunset yellow 0.02 mg in tablets containing 2.5 mg and 5 mg of lisinopril; azorubine dye 0.02 mg in tablets containing 10 mg of lisinopril; titanium dioxide 0.02 mg in tablets containing 20 mg of lisinopril).
Contraindications
Contraindications
– hypersensitivity to lisinopril, to other ACE inhibitors and components of the drug;
– history of angioedema, incl. against the background of the use of ACE inhibitors;
– hereditary angioedema or idiopathic edema;
– pregnancy;
– lactation period;
– age under 18 years (efficacy and safety have not been established);
– lactose intolerance, lactase deficiency, glucose-galactose malabsorption.
With caution
Aortic stenosis, hypertrophic obstructive cardiomyopathy, bilateral renal artery stenosis, stenosis of the artery of a single kidney, condition after kidney transplantation, severe renal failure (creatinine clearance (CC) less than 30 ml/min.), primary hyperaldosteronism, arterial hypotension, suppression of bone marrow hematopoiesis, hyponatremia (increased risk of developing arterial hypotension in patients on a low-salt or salt-free diet), hypovolemic conditions (including diarrhea, vomiting), systemic connective tissue diseases (including systemic lupus erythematosus, scleroderma), diabetes mellitus, hyperkalemia, coronary heart disease, cerebrovascular diseases (including cerebrovascular insufficiency), severe chronic heart failure, hemodialysis using high-flow dialysis membranes (AN69®), elderly.
Side Effects
Side Effects
The most common side effects: dizziness, headache, fatigue, diarrhea, dry cough, nausea.
From the cardiovascular system: marked decrease in blood pressure, chest pain, orthostatic hypotension, tachycardia, bradycardia, appearance or worsening of symptoms of heart failure, impaired atrioventricular conduction, myocardial infarction, palpitations.
From the central nervous system: emotional lability, impaired concentration, paresthesia, drowsiness, convulsive twitching of the muscles of the limbs and lips, increased fatigue, asthenic syndrome, confusion.
From the hematopoietic organs: leukopenia, neutropenia, agranulocytosis, thrombocytopenia, anemia (decreased concentration of hemoglobin, hematocrit, erythropenia).
From the respiratory system: dyspnea, dry cough, bronchospasm.
From the digestive system: dryness of the oral mucosa, anorexia, dyspepsia, changes in taste, abdominal pain, pancreatitis, jaundice (hepatocellular or cholestatic), hepatitis.
From the skin: increased sweating, alopecia, photosensitivity, itching.
From the genitourinary system: impaired renal function, oliguria, anuria, acute renal failure, uremia, proteinuria, decreased potency.
Allergic reactions: angioedema of the face, extremities, lips, tongue, epiglottis and/or larynx, skin rashes, urticaria, fever, positive test results for antinuclear antibodies, increased ESR, eosinophilia, leukocytosis, intestinal angioedema.
Other: myalgia, arthralgia/arthritis, vasculitis.
With the simultaneous use of ACE inhibitors and gold preparations (sodium aurothiomalate) intravenously, a symptom complex has been described, including facial flushing, nausea, vomiting and arterial hypotension.
Laboratory indicators: hyperkalemia, hyponatremia, increased activity of “liver” transaminases, hyperbilirubinemia, hypercreatininemia, increased urea concentration.
Interaction
Interaction
When used simultaneously with potassium-sparing diuretics (spironolactone, triamterene, amiloride), potassium preparations, salt substitutes containing potassium, the risk of developing hyperkalemia increases, especially in patients with impaired renal function. Therefore, they can be prescribed together only on the basis of an individual doctor’s decision with regular monitoring of serum potassium levels and renal function.
When used simultaneously with beta-blockers, blockers of “slow” calcium channels, diuretics and other antihypertensive drugs, the antihypertensive effect of the drug is enhanced.
Lisinopril can be used simultaneously with beta-blockers, acetylsalicylic acid (in doses less than 300 mg/day), thrombolytics, nitrates.
When used simultaneously with vasodilators, barbiturates, phenothiazines, tricyclic antidepressants, ethanol, the antihypertensive effect of the drug is enhanced.
When used simultaneously with nonsteroidal anti-inflammatory drugs (NSAIDs) (including selective cyclooxygenase-2 inhibitors), estrogens, and adrenergic stimulants, the antihypertensive effect of lisinopril is reduced.
When used simultaneously with lithium preparations, the elimination of lithium from the body is slowed down (increased cardiotoxic and neurotoxic effects of lithium).
When used simultaneously with antacids and cholestyramine, absorption in the gastrointestinal tract is reduced.
The drug enhances the neurotoxicity of salicylates, weakens the effect of hypoglycemic agents for oral administration, norepinephrine (noradrenaline), epinephrine (adrenaline) and anti-gout drugs, enhances the effects (including side effects) of cardiac glycosides, the effect of peripheral muscle relaxants, reduces the excretion of quinidine.
Reduces the effect of oral contraceptives. When taking methyldopa simultaneously, the risk of hemolysis increases.
Concomitant use with selective serotonin reuptake inhibitors may lead to severe hyponatremia.
Combined use with allopurinol, procainamide, and cytostatics can lead to leukopenia.
With the simultaneous use of ACE inhibitors and gold preparations (sodium aurothiomalate) intravenously, a symptom complex has been described, including facial flushing, nausea, vomiting and arterial hypotension.
Overdose
Overdose
Symptoms:
marked decrease in blood pressure, dryness of the oral mucosa, drowsiness, urinary retention, constipation, water-electrolyte imbalance, renal failure, increased breathing, tachycardia, palpitations, bradycardia, dizziness, anxiety, increased irritability.
Treatment:
gastric lavage, taking activated charcoal, placing the patient in a horizontal position with raised legs, replenishing the volume of circulating blood – intravenously with 0.9% sodium chloride solution or other plasma-substituting solutions, if necessary – vasopressor drugs, monitoring the functions of the cardiovascular and respiratory systems, blood pressure, blood volume, urea, creatinine, water-electrolyte balance. In case of persistent bradycardia, use an artificial pacemaker.
Hemodialysis is effective.
Manufacturer
Manufacturer
Vertex, Russia
Additional information
| Manufacturer | Vertex, Russia |
|---|---|
| Medication form | pills |
| Brand | Vertex |
Other forms…
Related products
Buy Lisinopril-Vertex, tablets 20 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.