No products in the cart.
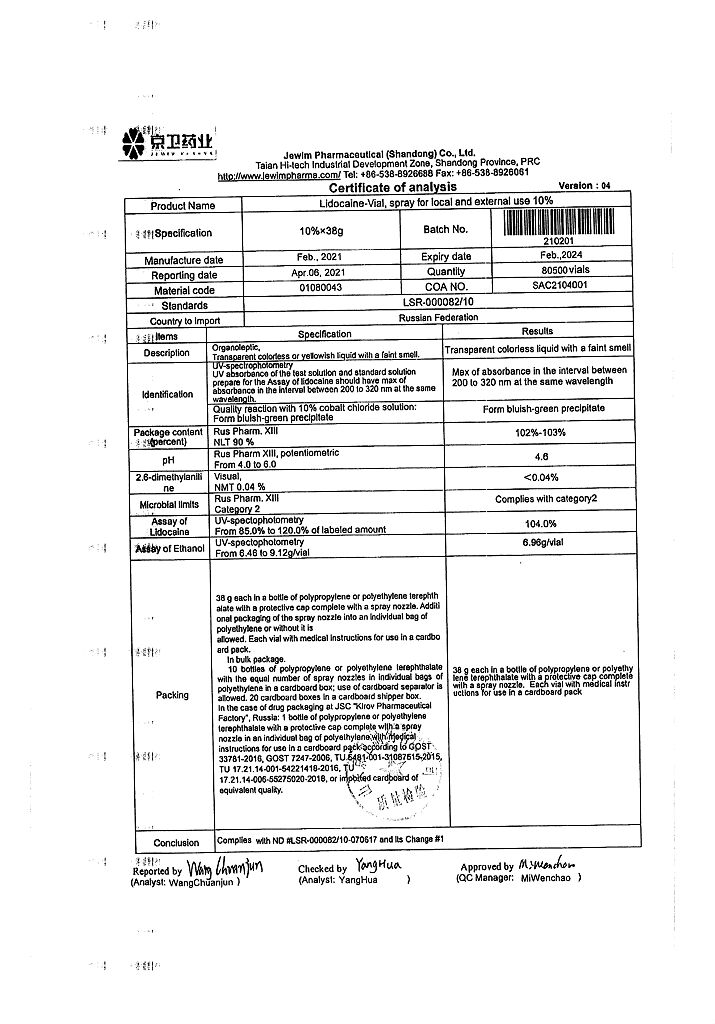
Lidocaine-Vial, 10% spray 38 g
€7.53 €6.59
EAN: 6921665004063
SKU: 223695
Categories: Anesthesia and resuscitation, Local anesthetics, Medicine
Description
Lidocaine is a local anesthetic and antiarrhythmic drug.
The antiarrhythmic activity is due to inhibition of phase 4 (diastolic depolarization) in Purkinje fibers, reduction of automaticity, suppression of ectopic foci of excitation. The rate of rapid depolarization (phase 0) is not affected or slightly reduced.
increases membrane permeability to potassium ions, accelerates the process of repolarization and shortens the action potential. It does not change the excitability of the sinus-atrial node and has little effect on conduction and myocardial contractility. When administered intravenously it works quickly and shortly (10-20 min).
The mechanism of local anesthetic effect consists in stabilization of neuronal membrane, reduction of its permeability to sodium ions, which prevents the emergence of action potential and impulse conduction. Antagonism with calcium ions is possible.
It is rapidly hydrolyzed in a weakly alkaline environment of tissues and after a short latent period acts for 60-90 minutes. In inflammation (tissue acidosis) anesthetic activity decreases.
Effective in all types of local anesthesia. It dilates the blood vessels. It has no irritating effect on the tissue.
Indications
Indications
The drug may be used for local anesthesia in the following cases:
Active ingredient
Active ingredient
Composition
Composition
Active ingredient:
Lidocaine 4.8 mg;
Excipients:
Ethanol (96%),
Pearmint oil,
Propylene glycol
How to take, the dosage
How to take, the dosage
The aerosol is sprayed onto mucous membranes.
In each spraying of 1 portion of the aerosol, 4.8 mg of lidocaine is released to the surface.
The dose depends on the indication and the surface area to be anesthetized. In order to avoid high plasma concentrations of lidocaine the lowest dose that provides a satisfactory effect should be used. Usually 1-3 sprays are sufficient, although 15-20 sprays are used in obstetrics (maximum dose is 40 sprays/70 kg body weight).
The drug can be applied to large surfaces using an impregnated swab.
In children under 2 years of age, the drug is preferably administered by swabbing to avoid the fear associated with spraying and the burning sensation.
For patients with hepatic and/or cardiac insufficiency, a 40% dose reduction is recommended.
When using the aerosol, the can should be held upright.
Interaction
Interaction
It is undesirable to combine lidocaine with the following drugs:
With beta-adrenoblockers because of increased toxic properties of lidocaine, with digitoxin because of weakened cardiotonic effect, with curare-like drugs because muscle relaxation is increased.
It is not rational to prescribe lidocaine together with aymalin, amiodarone, verapamil or quinidine due to increased cardiodepressant effect.
The combined use of lidocaine and novocainamide may cause CNS agitation and hallucinations.
When hexenal or thiopental sodium are administered intravenously against the background of lidocaine action, respiratory depression may occur.
Impact of MAO inhibitors may increase the local anesthetic effect of lidocaine. Patients taking MAO inhibitors should not administer lidocaine parenterally.
The simultaneous administration of lidocaine and polymyxin-B may increase the inhibitory effect on neuromuscular transmission, so the respiratory function of the patient should be monitored in this case.
The simultaneous use of lidocaine with hypnotics or sedatives may increase their CNS depressant effect. When lidocaine is administered intravenously to patients taking cimetidine such unwanted effects as stunned state, somnolence, bradycardia, parasthesias and others are possible. This is associated with increased plasma levels of lidocaine, which is explained by the release of lidocaine from bonding with blood proteins, as well as a slowdown of its inactivation in the liver. If combined therapy with these drugs is necessary, the dose of lidocaine should be reduced.
Pharmaceutical interaction
The following drugs increase the concentration of lidocaine in blood serum when used simultaneously: aminazin, cimetidine, propranolol, pethidine, bupivacaine, quinidine, disopyramide, amitriptyline, imipramine, nortriptyline.
Special Instructions
Special Instructions
Please note that children have a much more frequent swallowing reflex than adults.
Lidocaine aerosol is not recommended for local anesthesia before tonsillectomy and adenotomy in children under 8 years of age.
Contraindications
Contraindications
If plaster is used in dentistry as an impression material, aerosol is contraindicated because of the risk of aspiration.
Side effects
Side effects
Allergic reactions: rarely – rash, itching, exfoliative dermatitis, anaphylactic shock, hyperthermia.
Local reactions: a slight burning sensation, which disappears as the anesthetic effect develops (within 1 minute).
Overdose
Overdose
Symptoms: Increased sweating, pale skin, dizziness, headache, blurred vision, tinnitus, diplopia, decreased BP, bradycardia, arrhythmia, drowsiness, chills, numbness, tremor, anxiety, agitation, seizures, methemoglobinemia, cardiac arrest.
Treatment: In case of the first signs of intoxication (dizziness, nausea, vomiting, euphoria), further administration is stopped, the patient is transferred to a horizontal position; inhalation of oxygen is prescribed; in convulsions, IV 10 mg diazepam; in bradycardia, m-choline blockers (atropine), vasoconstrictors (norepinephrine, phenylephrine). Dialysis is ineffective.
Pregnancy use
Pregnancy use
Lidocaine aerosol can be used during pregnancy because it is not dangerous in the recommended doses.
It is not known whether lidocaine is excreted with the breast milk. Caution should be exercised when prescribing the drug to a nursing mother.
In lactation, the use of the drug is possible after careful assessment of the expected benefit to the mother and the potential risk to the infant.
Similarities
Similarities
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a light-protected place, at a temperature not exceeding 30 °C |
| Manufacturer | Jevim Pharmaceutical (Shandong) Co., China |
| Medication form | topical spray |
| Brand | Jevim Pharmaceutical (Shandong) Co. |
Related products
Buy Lidocaine-Vial, 10% spray 38 g with delivery to USA, UK, Europe and over 120 other countries.