No products in the cart.
Lantus SoloStar, 100 iu/ml 3 ml cartridges in syringe pens 5 pcs
€91.44 €76.20
Out of stock
(E-mail when Stock is available)
Description
Pharmacodynamics
Insulin Glargin is an analog of human insulin obtained by recombination of DNA-bacteria species of Escherichia coli (strain K12).
Insulin glargine is designed as an analogue of human insulin with low solubility in a neutral medium. In Lantus® SoloStar® it is fully soluble, which is ensured by the acidic reaction of the injection solution (pH 4). After injection into the subcutaneous fat, the acidic reaction of the solution is neutralized, resulting in the formation of microprecipitates from which small amounts of insulin glargin are continuously released, providing a predictable, smooth (no peaks) concentration-time curve profile and prolonged action of the drug.
Insulin glargine is metabolized to the two active metabolites M1 and M2 (see Pharmacokinetics).
Binding to insulin receptors: The kinetics of binding to specific insulin receptors in insulin glargin and its metabolites – M1 and M2 – are very similar to those of human insulin, so that insulin glargin is able to have a biological effect similar to that of endogenous insulin.
The most important action of insulin and its analogues, including insulin glargin, is regulation of glucose metabolism. Insulin and its analogues decrease blood glucose concentration by stimulating glucose uptake by peripheral tissues (especially skeletal muscle and adipose tissue) and inhibiting glucose formation in the liver.
Insulin suppresses lipolysis in adipocytes and inhibits proteolysis while increasing protein synthesis.
The prolonged action of insulin glargine is directly related to its reduced rate of absorption, which allows the drug to be used once a day. After p / n injection the onset of its action occurs on average in 1 hour. Average duration of action is 24 hours, maximum – 29 hours. Duration of action of insulin and its analogues such as insulin glargine may vary considerably in different persons or in the same person.
The efficacy of Lantus® SoloStar® has been shown in children over 2 years of age with type 1 diabetes. And in children aged 2-6 years, the incidence of hypoglycemia with clinical manifestations when using insulin glargine was lower both during the day and at night compared to the use of insulin isophan (respectively, an average of 25.5 episodes versus 33 episodes per patient for one year). In a five-year follow-up of patients with type 2 diabetes, there were no significant differences in the progression of diabetic retinopathy with treatment with insulin glargine compared to insulin isophane.
Binding to insulin-like growth factor 1 (IGF-1) receptors: The affinity of insulin glargine for the IGF-1 receptor is about 5-8 times higher than that of human insulin (but about 70-80 times lower than that of IGF-1), while, compared with human insulin, insulin glargine metabolites M1 and M2 have slightly lower affinity for the IGF-1 receptor.
The total therapeutic concentration of insulin (insulin glargine and its metabolites) determined in patients with type 1 diabetes was markedly lower than the concentration required for half-maximal binding to IGF-1 receptors and subsequent activation of the mitogen-proliferative pathway triggered via IGF-1 receptors. Physiological concentrations of endogenous IGF-1 can activate the mitogen-proliferative pathway; however, therapeutic insulin concentrations determined with insulin therapy, including treatment with Lantus® SoloStar®, are significantly lower than the pharmacological concentrations necessary to activate the mitogen-proliferative pathway.
The ORIGIN (Outcome Reduction with Initial Glargine INtervention) study was an international, multicenter, randomized study conducted in 12537 patients at high risk for cardiovascular disease and with impaired fasting glycemia (LPG), impaired glucose tolerance (IGT) or early-stage type 2 diabetes. Study participants were randomized into groups (1:1): a group of patients receiving insulin glargine (n=6264), which was titrated until reaching a fasting blood glucose concentration (BGC) ≤5.3 mmol, and a group of patients receiving standard treatment (n=6273). The first endpoint of the study represented the time to development of cardiovascular death, the first development of nonfatal myocardial infarction or nonfatal stroke, and the second endpoint represented the time to the first occurrence of any of the complications listed above or to the revascularization procedure (coronary, carotid or peripheral arteries) or to hospitalization for the development of heart failure.
The secondary endpoints were all-cause mortality and combined microvascular outcomes. The ORIGIN study showed that treatment with insulin glargine compared with standard hypoglycemic therapy did not alter the risk of cardiovascular complications or cardiovascular mortality; there were no differences in any component of the end points, all-cause mortality, combined microvascular outcome measures.
At baseline, the median HbA1c value was 6.4%. Median HbA1c values during treatment ranged from 5.9-6.4% in the insulin glargine group and 6.2-6.6% in the standard treatment group over the entire follow-up period. The incidence of severe hypoglycemia was 1.05 episodes per 100 patient-years of therapy in the patient group receiving insulin glargine and 0.3 episodes per 100 patient-years of therapy in the patient group receiving standard hypoglycemic therapy. The incidence of nonsevere hypoglycemia was 7.71 episodes per 100 patient-years of therapy in the group of patients receiving insulin glargine and 2.44 episodes per 100 patient-years of therapy in the group of patients receiving standard hypoglycemic therapy. In the 6-year study, 42% of patients in the group receiving insulin glargine had no episodes of hypoglycemia.
The median change in body weight versus outcome at the last treatment visit was 2.2 kg higher in the insulin glargine group than in the standard treatment group.
Pharmacokinetics
. A comparative study of plasma concentrations of insulin glargine and insulin isophan in healthy subjects and patients with diabetes mellitus after a single injection revealed slower and significantly longer absorption and no peak concentration in insulin glargine compared to insulin isophan. With a single daily infusion of Lantus® SoloStar®, the Css of insulin glargine in the blood is reached after 2-4 days with daily infusion.
In intravenous administration, the T1/2 of insulin glargine and human insulin were comparable. No significant differences in serum insulin concentrations were found when insulin glargine was injected into the abdomen, upper arm or thigh. Compared with human insulin of medium duration of action, insulin glargine is characterized by less variability in pharmacokinetic profile, both in the same and in different patients. In humans, insulin glargin is partially cleaved at the carboxyl end (C-end) side of the β-chain (beta-chain) to form two active metabolites M1 (21A G1u-insulin) and M2 (21A G1u-des-30B-Thg-insulin) in the subcutaneous fatty tissue. Metabolite M1 predominantly circulates in blood plasma. Systemic exposure to M1 metabolite increases with increasing drug dose.
Comparing pharmacokinetic and pharmacodynamic data showed that the drug action is mainly due to systemic exposure of M1 metabolite. In the vast majority of patients, insulin glargine and M2 metabolite could not be detected in the systemic bloodstream. In cases where insulin glargine and M2 metabolite were detected in the blood, their concentrations were independent of the dose of Lantus® SoloStar® administered.
Special patient groups
Age and sex. There is no information on the effect of age and sex on the pharmacokinetics of insulin glargine. However, these factors did not cause differences in the safety and efficacy of the drug.
Smoking. In clinical trials, subgroup analysis showed no differences in safety and efficacy of insulin glargine for this patient group compared to the general population.
Obesity. No differences in safety and efficacy of insulin glargine and insulin isophan were shown in obese patients compared to patients with normal body weight.
Children. In children with type 1 diabetes between 2 and 6 years of age, plasma concentrations of insulin glargine and its major metabolites M1 and M2 before the next dose were similar to those in adults, indicating no accumulation of insulin glargine and its metabolites with continued use of insulin glargine in children.
Indications
Indications
Diabetes mellitus requiring insulin treatment in adults, adolescents and children over 2 years of age.
Pharmacological effect
Pharmacological effect
Pharmacodynamics
Insulin glargine is an analogue of human insulin, obtained by recombination of DNA bacteria of the species Escherichia coli (strains K12).
Insulin glargine was developed as an analogue of human insulin, characterized by low solubility in a neutral environment. As part of the drug Lantus® SoloStar®, it is completely soluble, which is ensured by the acidic reaction of the injection solution (pH 4). After injection into the subcutaneous fat, the acidic reaction of the solution is neutralized, which leads to the formation of microprecipitates, from which small amounts of insulin glargine are constantly released, providing a predictable, smooth (no peaks) profile of the concentration-time curve, as well as prolonged action of the drug.
Insulin glargine is metabolized to two active metabolites M1 and M2 (see Pharmacokinetics).
Binding to insulin receptors: The binding kinetics of insulin glargine and its metabolites M1 and M2 to specific insulin receptors is very similar to that of human insulin, and therefore insulin glargine is capable of exerting biological effects similar to those of endogenous insulin.
The most important effect of insulin and its analogues, incl. and insulin glargine, is the regulation of glucose metabolism. Insulin and its analogues reduce blood glucose concentrations by stimulating glucose uptake into peripheral tissues (especially skeletal muscle and adipose tissue) and inhibiting glucose production in the liver.
Insulin suppresses lipolysis in adipocytes and inhibits proteolysis, while simultaneously increasing protein synthesis.
The prolonged action of insulin glargine is directly related to the reduced rate of its absorption, which allows the drug to be used once a day. After subcutaneous administration, the onset of action occurs on average after 1 hour. The average duration of action is 24 hours, the maximum is 29 hours. The duration of action of insulin and its analogues, such as insulin glargine, can vary significantly between different individuals or within the same person.
The effectiveness of the use of the drug Lantus® SoloStar® has been shown in children over 2 years of age with type 1 diabetes mellitus. Moreover, in children in the age group 2–6 years, the incidence of hypoglycemia with clinical manifestations when using insulin glargine was lower both during the day and at night compared to the use of isophane insulin (respectively, an average of 25.5 episodes versus 33 episodes per patient for one year). During a five-year follow-up of patients with type 2 diabetes, there were no significant differences in the progression of diabetic retinopathy when treated with insulin glargine compared with isophane insulin.
Association with insulin-like growth factor 1 (IGF-1) receptors: the affinity of insulin glargine for the IGF-1 receptor is approximately 5-8 times higher than that of human insulin (but approximately 70-80 times lower than that of IGF-1), while, compared with human insulin, the insulin glargine metabolites M1 and M2 have slightly higher affinities for the IGF-1 receptor less.
The total therapeutic concentration of insulin (insulin glargine and its metabolites) determined in patients with type 1 diabetes was markedly lower than the concentration required for half-maximal binding to IGF-1 receptors and subsequent activation of the mitogenic-proliferative pathway triggered through IGF-1 receptors. Physiological concentrations of endogenous IGF-1 can activate the mitogenic-proliferative pathway; however, therapeutic concentrations of insulin determined during insulin therapy, including treatment with Lantus® SoloStar®, are significantly lower than the pharmacological concentrations required to activate the mitogenic-proliferative pathway.
The ORIGIN (Outcome Reduction with Initial Glargine INtervention) study was an international, multicenter, randomized study conducted in 12,537 patients at high risk of developing cardiovascular disease and impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or early stage type 2 diabetes mellitus. Study participants were randomized into groups (1:1): a group of patients receiving insulin glargine (n=6264), which was titrated to achieve a fasting blood glucose (FBG) concentration of ≤5.3 mmol, and a group of patients receiving standard treatment (n=6273). The first endpoint of the study was the time to the first occurrence of cardiovascular death, the first occurrence of nonfatal myocardial infarction, or the first nonfatal stroke, and the second endpoint was the time to the first occurrence of any of the above complications or to a revascularization procedure (coronary, carotid, or peripheral artery), or to hospitalization for the development of heart failure.
Secondary endpoints were all-cause mortality and a composite measure of microvascular outcomes. The ORIGIN study showed that treatment with insulin glargine, compared with standard hypoglycemic therapy, did not change the risk of cardiovascular events or cardiovascular mortality; There were no differences in any of the endpoints, all-cause mortality, or the composite microvascular outcome measure.
At baseline, the median HbA1c value was 6.4%. Median HbA1c values during treatment ranged from 5.9–6.4% in the insulin glargine group and 6.2–6.6% in the standard treatment group throughout the follow-up period. In the group of patients receiving insulin glargine, the incidence of severe hypoglycemia was 1.05 episodes per 100 patient-years of therapy, and in the group of patients receiving standard hypoglycemic therapy – 0.3 episodes per 100 patient-years of therapy. The incidence of non-severe hypoglycemia in the group of patients receiving insulin glargine was 7.71 episodes per 100 patient-years of therapy, and in the group of patients receiving standard hypoglycemic therapy – 2.44 episodes per 100 patient-years of therapy. In a 6-year study, 42% of patients in the insulin glargine group did not experience any hypoglycemia.
The median change in body weight from outcome at the last treatment visit was 2.2 kg higher in the insulin glargine group than in the standard care group.
Pharmacokinetics
A comparative study of the concentrations of insulin glargine and insulin isophane in blood plasma in healthy people and patients with diabetes mellitus after subcutaneous administration of the drugs revealed a slower and significantly longer absorption, as well as the absence of a concentration peak for insulin glargine compared to insulin isophane. With a single subcutaneous administration of the drug Lantus® SoloStar® during the day, Css insulin glargine in the blood is achieved after 2–4 days with daily administration.
With intravenous administration, T1/2 of insulin glargine and human insulin were comparable. When insulin glargine was administered to the abdomen, shoulder, or thigh, no significant differences in serum insulin concentrations were found. Compared with intermediate-acting human insulin, insulin glargine has less variability in its pharmacokinetic profile, both within the same and between patients. In humans, in the subcutaneous fat tissue, insulin glargine is partially cleaved from the carboxyl end (C-terminus) of the β-chain (beta chain) to form two active metabolites M1 (21A G1y-insulin) and M2 (21A G1y-des-30B-Thr-insulin). The M1 metabolite circulates predominantly in the blood plasma. Systemic exposure of the M1 metabolite increases with increasing dosage of the drug.
A comparison of pharmacokinetics and pharmacodynamics data showed that the effect of the drug is mainly due to the systemic exposure of the M1 metabolite. In the vast majority of patients, insulin glargine and the M2 metabolite could not be detected in the systemic circulation. In cases where it was nevertheless possible to detect insulin glargine and the M2 metabolite in the blood, their concentrations did not depend on the administered dose of Lantus® SoloStar®.
Special patient groups
Age and gender. There is no information on the effect of age and gender on the pharmacokinetics of insulin glargine. However, these factors did not cause differences in the safety and effectiveness of the drug.
Smoking. In clinical trials, subgroup analyzes showed no differences in the safety and effectiveness of insulin glargine in this group of patients compared with the general population.
Obesity. No differences in the safety and effectiveness of insulin glargine and isophane insulin have been shown in obese patients compared with normal weight patients.
Children. In children with type 1 diabetes mellitus aged 2 to 6 years, the concentrations of insulin glargine and its main metabolites M1 and M2 in the blood plasma before the next dose were similar to those in adults, which indicates the absence of accumulation of insulin glargine and its metabolites with constant use of insulin glargine in children.
Special instructions
Special instructions
Lantus® SoloStar® is not the drug of choice for the treatment of diabetic ketoacidosis. In such cases, intravenous administration of short-acting insulin is recommended.
Due to limited experience with the use of Lantus® SoloStar®, it was not possible to evaluate its effectiveness and safety in the treatment of patients with impaired liver function or with moderate or severe renal failure.
In patients with impaired renal function, the need for insulin may be reduced due to a slower elimination of insulin. In elderly patients, progressive deterioration of renal function may lead to a persistent decrease in insulin requirements.
In patients with severe liver failure, the need for insulin may be reduced due to a decrease in the ability to gluconeogenesis and a slowdown in the biotransformation of insulin.
In case of insufficient control of blood glucose levels, as well as in the presence of a tendency to develop hypo- or hyperglycemia, before proceeding with the correction of the dosage regimen, you should check the accuracy of the prescribed treatment regimen, compliance with the instructions regarding the drug administration sites and the correct technique of subcutaneous injections, taking into account all factors influencing this.
Hypoglycemia
The time for the development of hypoglycemia depends on the action profile of the insulins used and may thus change when changing the treatment regimen. Due to the increase in the time of entry into the body of long-acting insulin, when using the drug Lantus® SoloStar®, one should expect a lower likelihood of developing nocturnal hypoglycemia, whereas in the early morning hours this likelihood of developing hypoglycemia is higher. If hypoglycemia occurs in patients receiving Lantus® SoloStar®, the possibility of slowing recovery from hypoglycemia due to the prolonged action of insulin glargine should be taken into account.
Patients in whom episodes of hypoglycemia may be of particular clinical significance, such as patients with significant stenosis of the coronary arteries or cerebral vessels (risk of developing cardiac and cerebral complications of hypoglycemia), as well as patients with proliferative retinopathy, especially if they are not receiving photocoagulation treatment (risk of transient visual loss following hypoglycemia), should use special caution and intensify monitoring of glucose levels in blood.
Patients should be warned about conditions in which the warning symptoms of hypoglycemia may decrease. In patients at certain risk groups, symptoms of hypoglycemia may change, become less pronounced, or be absent. These include:
– patients whose blood glucose regulation has significantly improved;
– patients in whom hypoglycemia develops gradually;
– elderly patients;
– patients switched from animal insulin to human insulin;
– patients with neuropathy;
– patients with a long history of diabetes mellitus;
– patients suffering from mental disorders;
– patients receiving concomitant treatment with other drugs (see “Interaction”).
Such situations may result in severe hypoglycemia (with possible loss of consciousness) before the patient is aware that he is developing hypoglycemia.
If normal or reduced levels of glycosylated Hb are observed, it is necessary to consider the possibility of developing repeated unrecognized episodes of hypoglycemia (especially at night).
Patients’ compliance with the dosage regimen and diet, correct administration of insulin and knowledge of the warning signs of hypoglycemia help to significantly reduce the risk of developing hypoglycemia.
Factors that increase the tendency to hypoglycemia, the presence of which require particularly careful monitoring and may require adjustment of the insulin dose:
– changing the site of insulin administration;
– increasing sensitivity to insulin (for example, when eliminating stress factors);
– unusual, increased or prolonged physical activity;
– intercurrent diseases accompanied by vomiting, diarrhea;
– violation of diet and nutrition;
– missed meals;
– alcohol consumption;
– some uncompensated endocrine disorders (for example, hypothyroidism, insufficiency of the adenohypophysis or adrenal cortex);
– concomitant treatment with certain drugs (see “Interaction”).
Intercurrent diseases
Intercurrent illnesses require more intensive control of blood glucose levels. In many cases, an analysis for the presence of ketone bodies in the urine is indicated, and adjustment of the insulin dosage regimen is also often required. The need for insulin often increases. People with type 1 diabetes should continue to regularly consume at least a small amount of carbohydrates, even if they are able to eat only small amounts or cannot eat at all, or are vomiting, etc., and they should never stop taking insulin altogether.
Recommendations for handling the drug
When storing Lantus® SoloStar® in the refrigerator, ensure that the containers do not come into direct contact with the freezer compartment or frozen packages.
Before first use, the Lantus® SoloStar® syringe pen must be kept at room temperature for 1–2 hours.
Used SoloStar® disposable syringe pens should be stored at a temperature not exceeding 30 °C and protected from exposure to light.
Do not refrigerate the pre-filled SoloStar® syringe pen.
The shelf life of the drug in the SoloStar® disposable syringe pen after the first use is 4 weeks. It is recommended to mark the date of first administration of the drug on the label.
Influence on the ability to drive vehicles and machinery. Patients’ ability to concentrate and respond quickly may be impaired by conditions such as hypoglycemia, hyperglycemia, or visual impairment. This may pose a risk in situations where these abilities are particularly important (for example, driving a car or operating other machinery). Patients are advised to take precautions to avoid the development of hypoglycemia when driving. This is especially important for those patients who have mild or no symptoms that are warning signs of developing hypoglycemia, or for patients with frequent episodes of hypoglycemia. Such circumstances should be taken into account when driving a car.
Active ingredient
Active ingredient
Insulin glargine
Composition
Composition
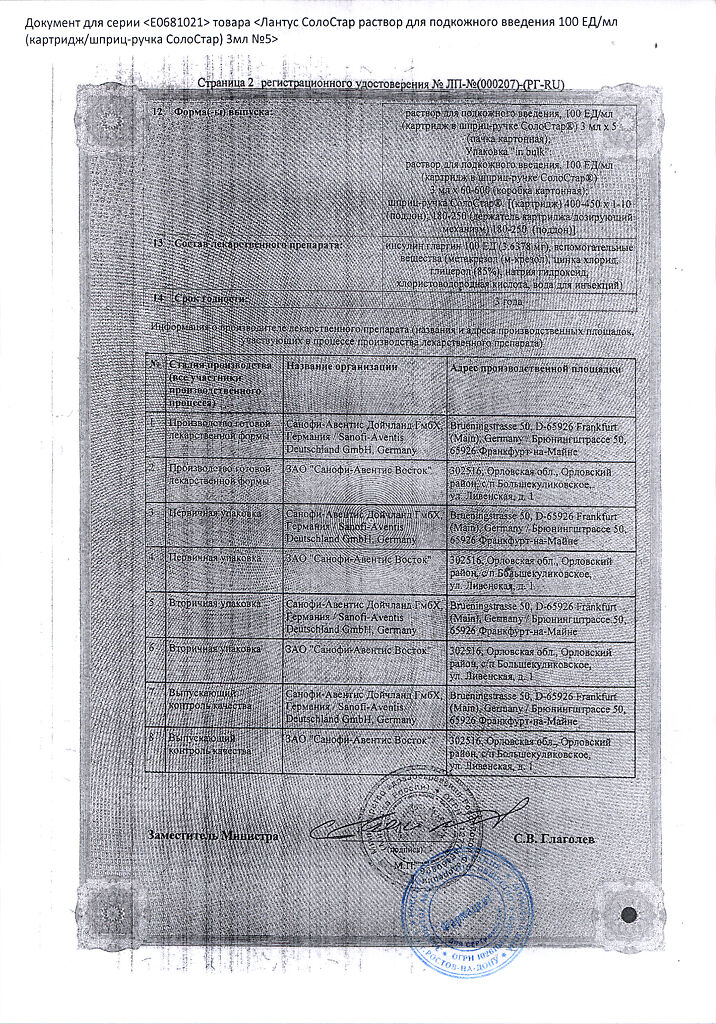
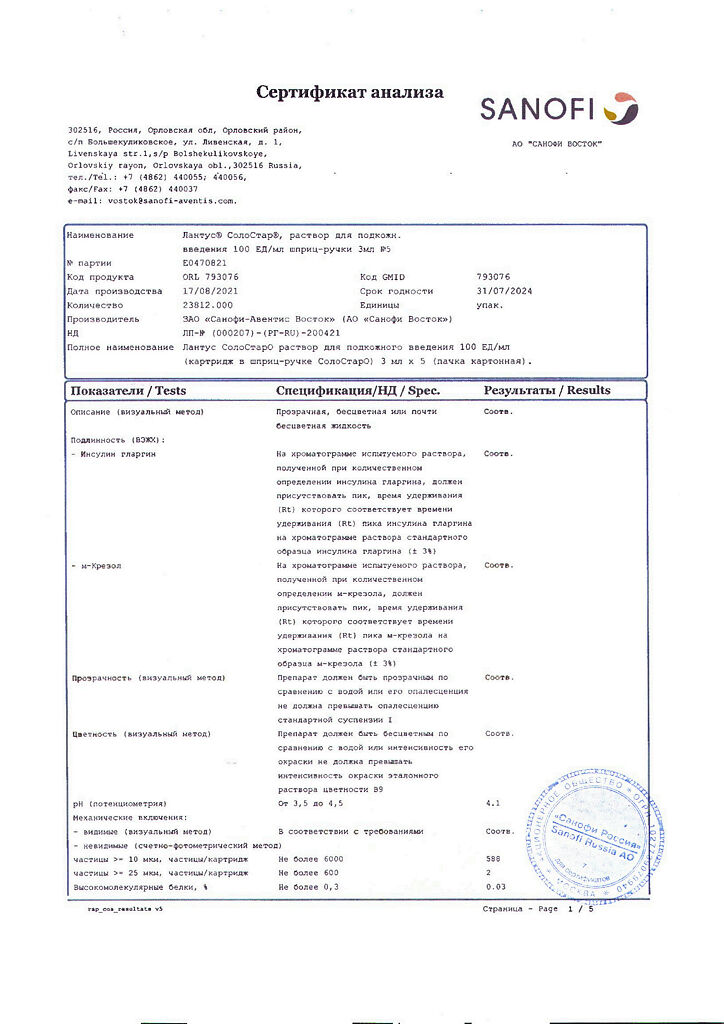
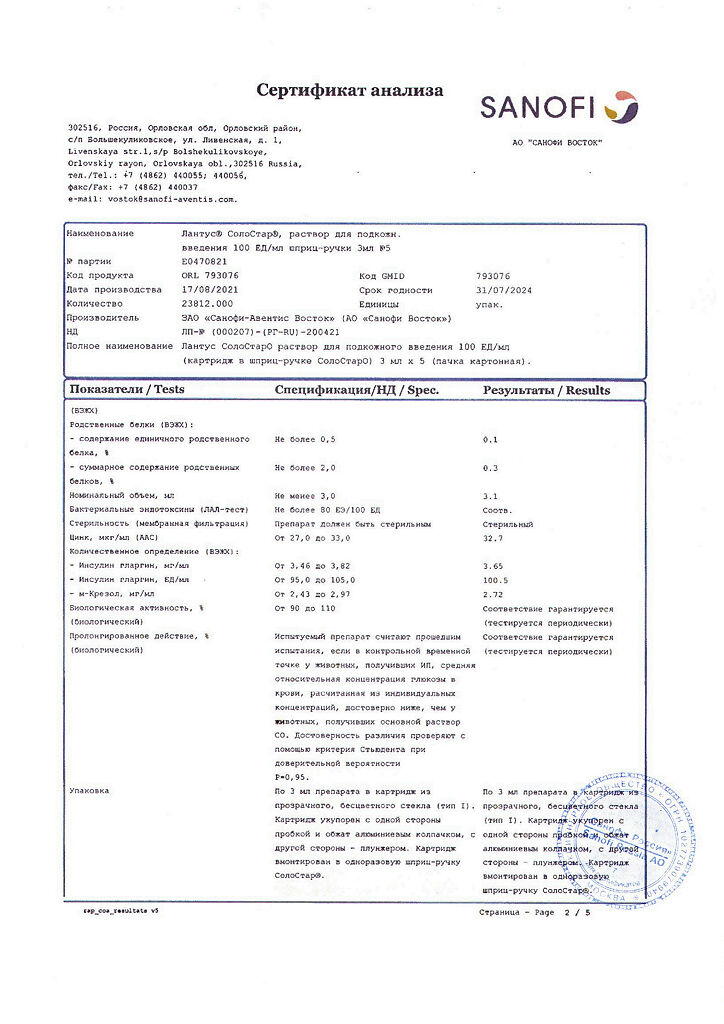
Active substance: insulin glargine 100 units (3.6378 mg)
Excipients: metacresol (m-cresol) – 2.7 mg; zinc chloride – 0.0626 mg (corresponds to 30 mcg of zinc); glycerol (85%) – 20 mg; sodium hydroxide – up to pH 4; hydrochloric acid – up to pH 4; water for injection – up to 1 ml.
Pregnancy
Pregnancy
Patients should inform their doctor about a current or planned pregnancy.
There are no randomized controlled clinical trials on the use of insulin glargine in pregnant women.
A large number of observations (more than 1000 pregnancy outcomes during retrospective and prospective observation) during the post-marketing use of insulin glargine showed the absence of any specific effects on the course and outcome of pregnancy or on the condition of the fetus or the health of newborns.
In addition, a meta-analysis of eight observational clinical trials including women who used insulin glargine (n=331) and insulin isophane (n=371) during pregnancy was conducted to evaluate the safety of insulin glargine and isophane insulin in pregnant women with pre-existing or gestational diabetes mellitus. This meta-analysis found no significant differences in maternal or neonatal safety between insulin glargine and isophane insulin during pregnancy.
No direct or indirect evidence of the embryotoxic or fetotoxic effects of insulin glargine has been obtained from animal studies.
For patients with pre-existing or gestational diabetes mellitus, it is important to maintain adequate metabolic regulation throughout pregnancy to prevent adverse outcomes associated with hyperglycemia.
The drug Lantus® SoloStar® can be used during pregnancy according to clinical indications.
The need for insulin may decrease in the first trimester of pregnancy and, in general, increase during the second and third trimesters.
Immediately after birth, the need for insulin decreases rapidly (the risk of hypoglycemia increases). In these conditions, careful monitoring of blood glucose concentrations is essential.
Patients during breastfeeding may require adjustments to their insulin dosage regimen and diet.
Contraindications
Contraindications
Hypersensitivity to insulin glargine or any of the auxiliary components of the drug;
Children under 2 years of age (no clinical data on use).
With caution: pregnant women (possibility of changes in insulin requirements during pregnancy and after childbirth).
Side Effects
Side Effects
The following undesirable effects are given by organ system in accordance with the following gradations of the frequency of their occurrence (according to the MedDRA classification): very often – ≥10%; often – ≥1–< 10%; uncommon - ≥0.1–<1%; rarely - ≥0.01–<0.1%; very rarely - ≤0.01%.
From the side of metabolism: very often – hypoglycemia. Hypoglycemia, the most common adverse reaction of insulin therapy, can occur when the dose of insulin is too high compared to the need.
Symptoms of hypoglycemia usually occur suddenly. However, often neuropsychiatric disorders associated with neuroglycopenia (feeling tired, unusual fatigue or weakness, decreased ability to concentrate, drowsiness, visual disturbances, headache, nausea, confusion or loss of consciousness, convulsive syndrome) are usually preceded by symptoms of adrenergic counterregulation (activation of the sympathoadrenal system in response to hypoglycemia) – hunger, irritability, nervous agitation or tremor, anxiety, pallor of the skin, cold sweat, tachycardia, severe palpitations (the faster hypoglycemia develops and the more severe it is, the more pronounced the symptoms of adrenergic counterregulation).
Attacks of severe hypoglycemia, especially repeated ones, can lead to damage to the nervous system. Episodes of prolonged and severe hypoglycemia can threaten the lives of patients, because With increasing hypoglycemia, even death is possible.
From the immune system: rarely – allergic reactions. Immediate allergic reactions to insulin are rare. Such reactions to insulin (including insulin glargine) or excipients may result in the development of generalized skin reactions, angioedema, bronchospasm, hypotension or shock and thus pose a threat to the patient’s life.
The use of insulin can cause the formation of antibodies to it. The formation of antibodies that cross-react with human insulin and insulin glargine occurs with equal frequency when using isophane insulin and insulin glargine. In rare cases, the presence of such antibodies to insulin may necessitate adjustment of the dosage regimen in order to eliminate the tendency to develop hypo- or hyperglycemia.
From the nervous system: very rarely – dysgeusia (impaired or distorted sense of taste).
From the organ of vision: rarely – visual impairment, retinopathy.
Significant changes in the regulation of blood glucose can cause temporary visual impairment due to changes in tissue turgor and the refractive index of the eye lens.
Long-term normalization of blood glucose reduces the risk of progression of diabetic retinopathy. Insulin therapy, accompanied by sharp fluctuations in blood glucose levels, may be accompanied by a temporary worsening of diabetic retinopathy. In patients with proliferative retinopathy, especially those not receiving photocoagulation treatment, episodes of severe hypoglycemia may lead to transient vision loss.
From the skin and subcutaneous fat: often – lipodystrophy (in 1-2% of patients). As with treatment with any other insulin preparations, lipodystrophy may develop at the injection site, which can slow down the local absorption of insulin; infrequently – lipoatrophy. Constantly changing injection sites within areas of the body recommended for subcutaneous insulin administration may help reduce the severity of this reaction or prevent its development.
From the musculoskeletal system and connective tissue: very rarely – myalgia.
General disorders and reactions at the injection site: often – reactions at the injection site (3-4%) (redness, pain, itching, urticaria, swelling or inflammation). Most minor insulin injection site reactions usually resolve within a few days to a few weeks; rarely – sodium retention, edema (especially if intensified insulin therapy leads to improvement of previously insufficient metabolic control).
The safety profile for patients under 18 years of age is generally similar to that for patients over 18 years of age. In patients under 18 years of age, reactions at the injection site and skin reactions (rash, urticaria) are relatively more common.
There are no safety data in patients under 2 years of age.
Interaction
Interaction
Oral hypoglycemic agents, ACE inhibitors, disopyramide, fibrates, fluoxetine, MAO inhibitors, pentoxifylline, propoxyphene, salicylates and sulfonamide antimicrobial agents may enhance the hypoglycemic effect of insulin and increase the susceptibility to the development of hypoglycemia. Concomitant use with insulin glargine may require adjustment of the insulin dose.
GCS, danazol, diazoxide, diuretics, glucagon, isoniazid, estrogens and gestagens (for example, in hormonal contraceptives), phenothiazine derivatives, somatotropin, sympathomimetics (for example, epinephrine, salbutamol, terbutaline) and thyroid hormones, protease inhibitors, atypical antipsychotics (for example, olanzapine or clozapine) – may weaken the hypoglycemic effect of insulin. Concomitant use with insulin glargine may require dose adjustment of insulin glargine.
Beta-blockers, clonidine, lithium salts or alcohol – it is possible to either enhance or weaken the hypoglycemic effect of insulin.
Pentamidine – when combined with insulin, can cause hypoglycemia, which sometimes gives way to hyperglycemia.
Sympatholytic drugs, such as beta-blockers, clonidine, guanethidine, and reserpine, may have decreased or absent signs of adrenergic counterregulation (activation of the sympathetic nervous system) when hypoglycemia develops.
Pharmaceutical interactions
When mixing Lantus® SoloStar® with other medicinal substances, incl. and other insulins, as well as dilution of the drug, the formation of a sediment or a change in the profile of the drug’s action over time is possible.
Overdose
Overdose
An overdose of insulin can lead to severe and sometimes prolonged hypoglycemia, which threatens the patient’s life.
Treatment: Episodes of mild hypoglycemia are usually controlled by ingestion of rapidly digestible carbohydrates. It may be necessary to change the drug dosage regimen, diet, or physical activity.
Episodes of more severe hypoglycemia, manifested by coma, seizures or neurological disorders, require intramuscular or subcutaneous administration of glucagon, as well as intravenous administration of a concentrated solution of dextrose (glucose). Long-term intake of carbohydrates and specialist supervision may be required, because After visible clinical improvement, relapse of hypoglycemia is possible.
Storage conditions
Storage conditions
In a place protected from light, at a temperature of 2–8 °C (do not freeze). After use, store at a temperature not exceeding 25 °C in cardboard packaging (but not in the refrigerator).
Keep out of the reach of children.
Shelf life
Shelf life
3 years. After opening – 4 weeks.
Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
Sanofi-Vostok JSC, Russia
Additional information
| Shelf life | 3 years. After opening – 4 weeks. Do not use after the expiration date stated on the package. |
|---|---|
| Conditions of storage | Store in a light-protected place at 2-8 °C (do not freeze). After use, store at a temperature not higher than 25 °C in a cardboard box (but not in the refrigerator). Keep out of the reach of children. |
| Manufacturer | Sanofi-Vostok JSC, Russia |
| Medication form | solution |
| Brand | Sanofi-Vostok JSC |
Related products
Buy Lantus SoloStar, 100 iu/ml 3 ml cartridges in syringe pens 5 pcs with delivery to USA, UK, Europe and over 120 other countries.