No products in the cart.
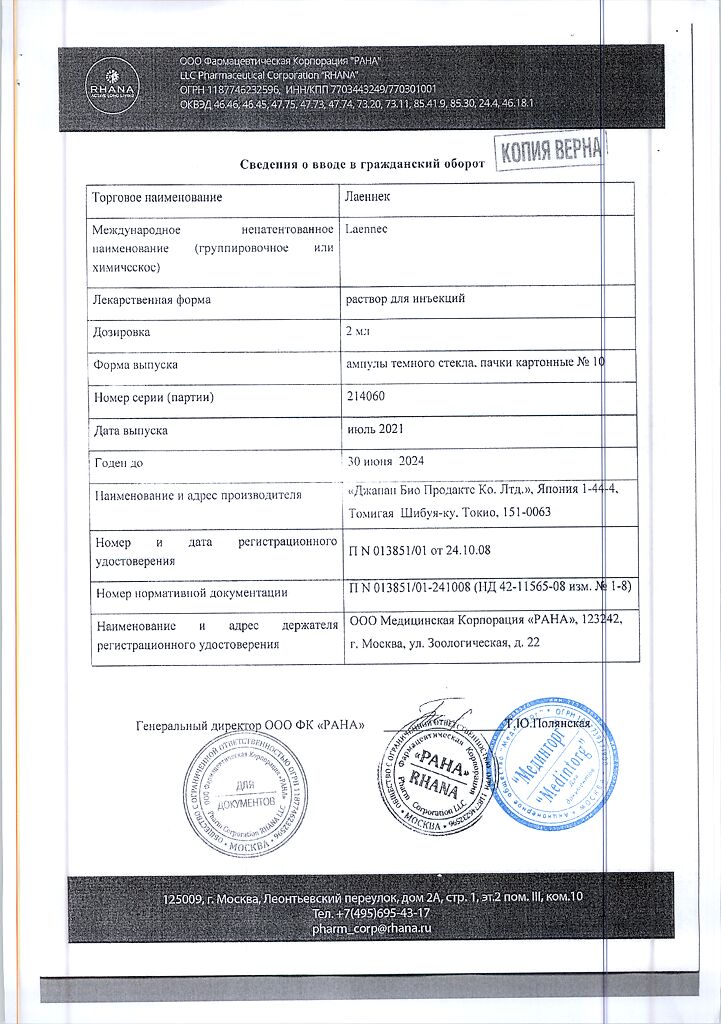
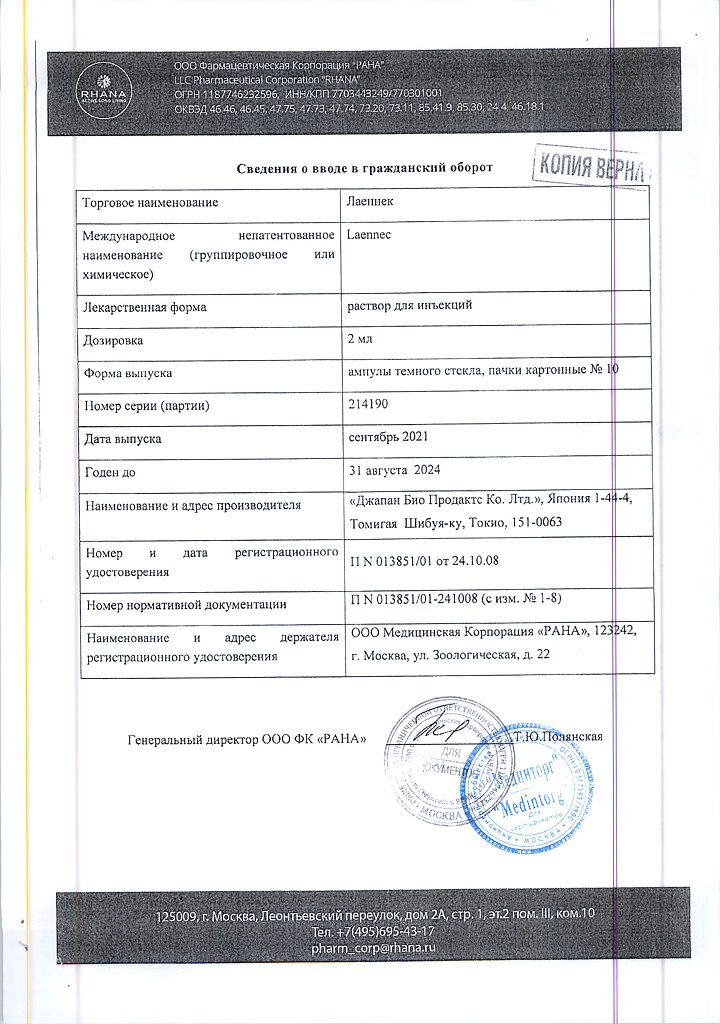
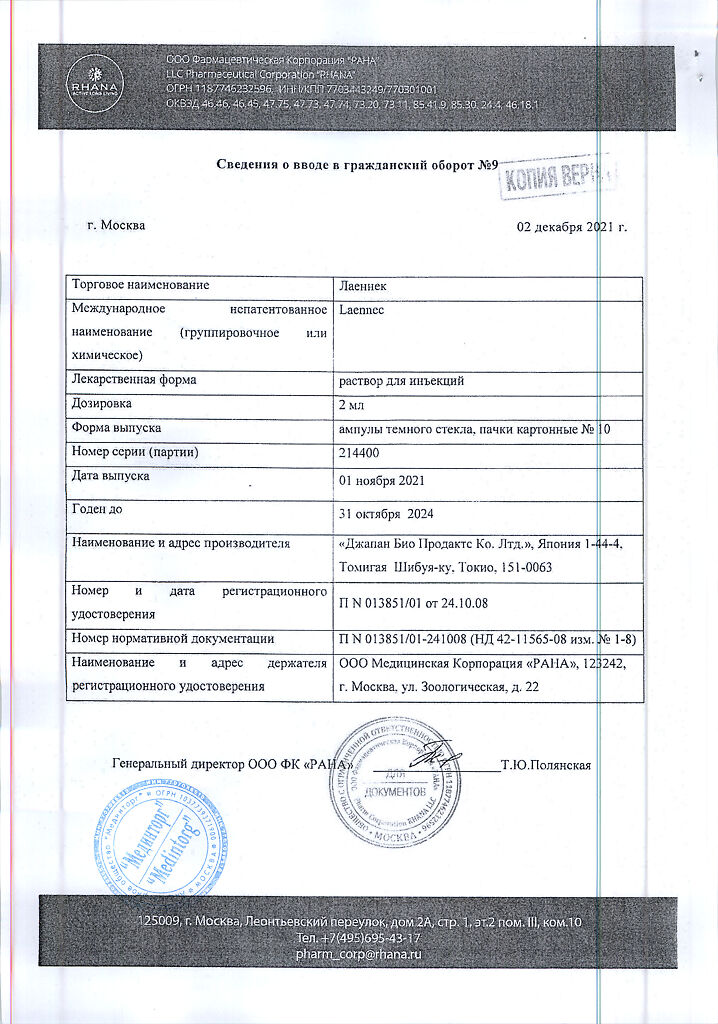
Laennec, 2 ml 10 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Laennek is a new unique remedy, which promotes rejuvenation of the body and restoration of health, is an immunomodulator, hepatoprotector, promotes immunity.
The preparation exhibits immunomodulatory properties due to the ability to stimulate humoral immunity and increase the functional activity of phagocytes and natural killers. It increases bactericidal activity of leukocytes of peripheral blood which shows its ability to destroy invaded pathogen. Cytokines contained in the drug activate the metabolic and supervising functions of skin cells.
The biologically active substances contained in the hydrolyzate stimulate the regeneration (proliferation) of hepatocytes, exhibit detoxifying properties, reduce deposition of lipids and cholesterol in liver cells, increase the activity of tissue respiration, activate metabolism in the liver, reduce the intensity of connective tissue in the liver.
Indications
Indications
chronic recurrent herpes (as part of complex therapy);
atopic dermatitis of moderate and severe severity, incl. complicated (as part of complex therapy);
chronic liver diseases: steatohepatitis (alcoholic, metabolic and mixed etiology) – as monotherapy.
Pharmacological effect
Pharmacological effect
Laennec is a new unique remedy that helps rejuvenate the body and restore health, is an immunomodulator, hepatoprotector, and helps improve immunity.
The drug exhibits immunomodulatory properties due to the ability to stimulate humoral immunity and increase the functional activity of phagocytes and natural killer cells. Increases the bactericidal activity of peripheral blood leukocytes, manifested in their ability to destroy the captured pathogen. The cytokines included in the drug activate the metabolic and supervisory functions of skin cells.
Biologically active substances contained in the hydrolyzate stimulate the regeneration (proliferation) of hepatocytes, exhibit detoxification properties, reduce the deposition of lipids and cholesterol in liver cells, increase the activity of tissue respiration, activate metabolism in the liver, and reduce the intensity of development of connective tissue in the liver.
Special instructions
Special instructions
According to currently available data, the drug can be prescribed to elderly people. However, given that physiological functions deteriorate in the elderly, the drug should be used under close supervision.
Use in pediatrics
Studies on the safety of Laennec in newborns (including premature infants) and minors have not been conducted. Use in children is not recommended.
Active ingredient
Active ingredient
Human placenta hydrolyzate
Composition
Composition
Solution for injection.
1 ampoule contains:
Active substance:
human placenta hydrolyzate 112 mg
Excipients:
water for injections,
sodium hydroxide or hydrochloric acid (to adjust pH).
Contraindications
Contraindications
childhood;
pregnancy;
lactation period;
hypersensitivity to the drug.
It should be used with caution in patients with polyvalent allergies to drugs and in the elderly.
Side Effects
Side Effects
Side effects are observed in 3.7% of patients.
Clinically significant adverse reactions: allergic reactions are possible.
Other adverse events: pain at the injection site (2.56%), allergic reactions (redness, itching) (0.37%), numbness at the injection site (0.37%), gynecomastia (0.37%) – a cause-and-effect relationship with the administration of the drug has not been established.
Interaction
Interaction
When mixing Laennec solution with other drugs that are strong bases (pH above 8.5), the activity of the drug is reduced.
To date, no other clinically significant drug interactions have been identified.
Overdose
Overdose
Currently, no cases of overdose with Laennec have been reported.
Storage conditions
Storage conditions
The drug should be stored in a place protected from light, out of reach of children, at a temperature of 18° to 25°C.
Shelf life
Shelf life
3 years.
Manufacturer
Manufacturer
Japan Bio Products Co., Ltd., Japan
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | The drug should be stored in the dark place out of the reach of children at 18 ° to 25 ° C. |
| Manufacturer | Japan Bio Products Co. |
| Medication form | solution for injection |
| Brand | #Н/Д |
Related products
Buy Laennec, 2 ml 10 pcs with delivery to USA, UK, Europe and over 120 other countries.