No products in the cart.
Description
Anti-allergic agent – stabilizer of mast cell membranes
ATC: S.01.G.X.08
Indications
Indications
Atopic bronchial asthma, hay fever (hay fever), allergic rhinitis, allergic conjunctivitis, atopic dermatitis, urticaria.
Pharmacological effect
Pharmacological effect
Antiallergic agent – mast cell membrane stabilizer
ATX: S.01.G.X.08
Special instructions
Special instructions
Abrupt cancellation of previous treatment with beta-adrenergic stimulants, glucocorticosteroids, ACTH in patients with bronchial asthma and bronchospastic syndrome after joining ketotifen therapy is not advisable; cancellation is carried out for at least 2 weeks, gradually reducing the dose.
Treatment is stopped gradually, over 2-4 weeks (relapse of asthmatic symptoms is possible).
For persons sensitive to sedation, the drug is prescribed in small doses in the first 2 weeks.
Not intended to relieve an attack of bronchial asthma.
In patients concomitantly receiving oral hypoglycemic agents, the platelet count in the peripheral blood should be monitored.
Effect on ability to drive:
Active ingredient
Active ingredient
Ketotifen
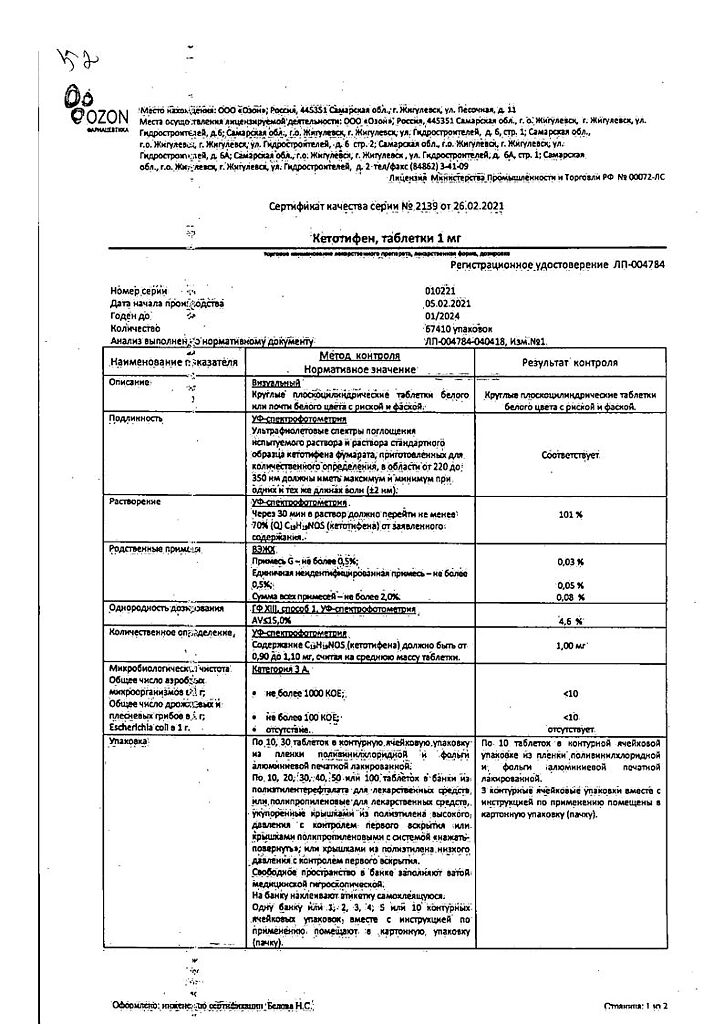
Composition
Composition
Composition per 1 tablet:
Active ingredient: ketotifen fumarate – 1.38 mg (corresponding to ketotifen – 1.00 mg);
Excipients:
microcrystalline cellulose – 112.22 mg,
calcium hydrogen phosphate – 20.00 mg,
corn starch – 5.00 mg,
magnesium stearate – 1.40 mg.
Contraindications
Contraindications
Hypersensitivity, pregnancy, lactation, children under 3 years of age.
With caution:
Epilepsy, liver failure.
Side Effects
Side Effects
From the nervous system: drowsiness, dizziness, slow reaction speed (disappear after a few days of therapy), sedation, feeling of fatigue; rarely – anxiety, sleep disturbances, nervousness (especially in children).
From the digestive system: dry mouth, increased appetite, nausea, vomiting, gastralgia, constipation.
From the urinary system: dysuria, cystitis.
Other: thrombocytopenia, weight gain, allergic skin reactions.
Interaction
Interaction
Strengthens the effect of sleeping pills, antihistamines, ethanol.
In combination with hypoglycemic drugs, the likelihood of developing thrombocytopenia increases.
Overdose
Overdose
Symptoms: drowsiness, confusion, disorientation, bradycardia or tachycardia, decreased blood pressure, shortness of breath, cyanosis, convulsions, increased excitability, coma.
Treatment: gastric lavage (if a little time has passed since administration), symptomatic treatment, with the development of convulsive syndrome – barbiturates or benzodiazepines. Dialysis is ineffective.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25°C.
Keep out of the reach of children.
Shelf life
Shelf life
3 years.
Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
Ozon, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date printed on the package. |
|---|---|
| Conditions of storage | In a dry place protected from light, at a temperature not exceeding 25 ° C. Store out of the reach of children. |
| Manufacturer | Ozon, Russia |
| Medication form | pills |
| Brand | Ozon |
Other forms…
Related products
Buy Ketotifen, tablets 1 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.