No products in the cart.
Description
Ketotifen has a pronounced antihistamine, weakly antiserotonin action, without anticholinergic effect. In higher doses it has a slightly depressing effect on the central nervous system.
Pharmacodynamics
The mechanism of action of Ketotifen is related to the ability to inhibit the release of histamine and other inflammatory mediators from mast cells due to membrane stabilization and also due to blockade of histamine H1 receptors. Ketotifen inhibits phosphodiesterase enzyme, as a result of which the level of cyclic adenosine monophosphate in mast cells increases.
The drug reduces or suppresses skin and bronchial reactions caused by the antigen, which leads to its use for prophylactic purposes. When used as monotherapy it does not stop asthma attacks, but leads to reduction of the number, duration and intensity of these attacks, and in some cases they completely disappear.
Limits eosinophilia, reduces the need and frequency of use of anti-asthmatic drugs: corticosteroids, bronchodilators, etc.
Pharmacokinetics
The drug is characterized by good and rapid resorption, without the danger of cumulation in the body. Bioavailability is approximately 50%, which is associated with metabolism during “first passage” through the liver. Time to reach maximum plasma concentration is 2-4 hours, and binding to plasma proteins is 75%. There are two phases of excretion, and most of the single dose is eliminated in the urine within 48 hours. Therapeutic effect occurs after 2 weeks of using the drug.
Indications
Indications
Atopic bronchial asthma, allergic rhinitis,
allergic conjunctivitis, hay fever (hay fever)
Ketotifen Sopharma effectively prevents attacks of bronchial asthma and other manifestations of immediate allergic reactions
Pharmacological effect
Pharmacological effect
Ketotifen has a pronounced antihistamine, weakly antiserotonin effect, without an anticholinergic effect. In higher doses it has a slightly depressant effect on the central nervous system.
Pharmacodynamics
The mechanism of action of Ketotifen is associated with the ability to inhibit the release of histamine and other inflammatory mediators from mast cells due to the stabilization of membranes, as well as due to the blockade of histamine H1 receptors. Ketotifen inhibits the enzyme phosphodiesterase, resulting in increased levels of cyclic adenosine monophosphate in mast cells.
The drug reduces or suppresses reactions of the skin and bronchi caused by the antigen, which determines its use for prophylactic purposes. When used as monotherapy, it does not relieve asthma attacks, but leads to a decrease in the number, duration and intensity of these attacks, and in some cases they completely disappear.
Reduces eosinophilia, reduces the need and frequency of use of antiasthmatic drugs: corticosteroids, bronchodilators, etc.
Pharmacokinetics
The drug is characterized by good and rapid resorption, without the danger of accumulation in the body. Bioavailability is approximately 50%, which is due to “first pass” metabolism through the liver. The time to reach maximum plasma concentration is 2-4 hours, and plasma protein binding is 75%. Excretion from the body occurs in two phases, and within 48 hours the main part of the taken single dose is excreted in the urine. The therapeutic effect develops after 2 weeks of the course of taking the drug.
Special instructions
Special instructions
Patients taking ketotifen should refrain from potentially hazardous activities that involve increased alertness (for example, driving a car). The drug can be used during lactation only if necessary, since it passes into breast milk.
Active ingredient
Active ingredient
Ketotifen
Composition
Composition
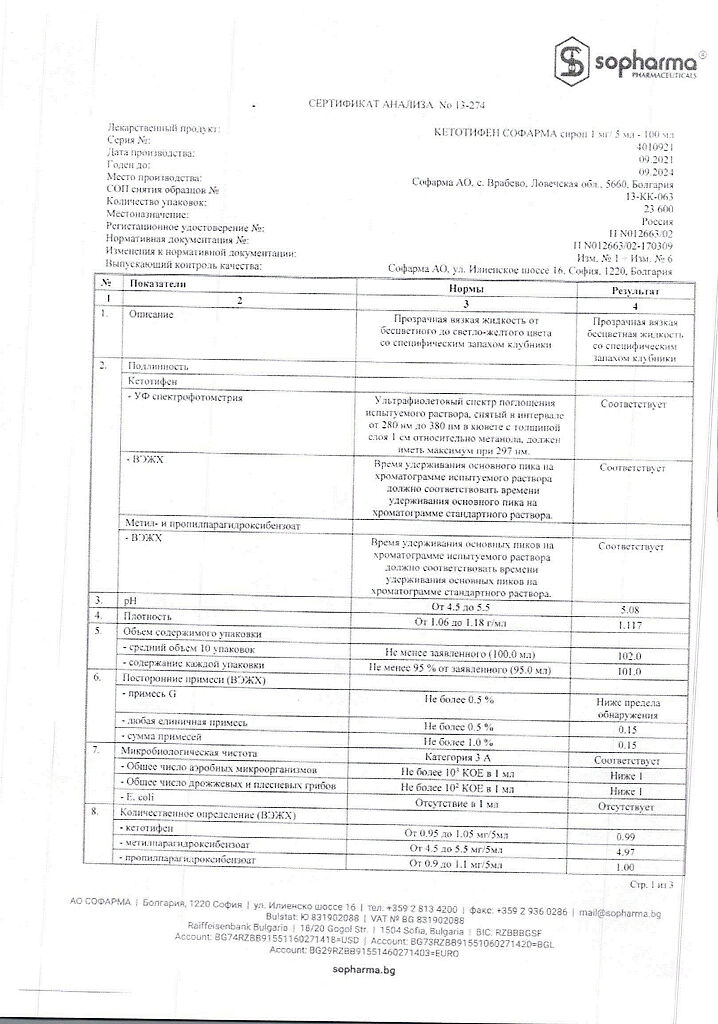
5 ml of syrup contains:
Pregnancy
Pregnancy
Although animal studies did not show any effect on pregnancy and peri- and postnatal development when Ketotifen was used at doses tolerated by animals, its safety in human pregnancy has not been established.
Therefore, during pregnancy, Ketotifen can be prescribed only in cases of extreme necessity.
It is believed that Ketotifen is also excreted in women’s breast milk, so mothers taking Ketotifen should not breastfeed their children.
Contraindications
Contraindications
There are no absolute contraindications.
The drug should not be taken during pregnancy, while taking oral antidiabetic drugs, as this may lead to thrombocytopenia; with sedatives and alcohol.
Hypersensitivity to the components of the drug.
Side Effects
Side Effects
In the first days, you may experience drowsiness, dry mouth, mild dizziness, fatigue, and slowed mental reactions, which usually disappear after a few days. It is recommended to use a lower dose (5 ml per day) during the first week. Less common is an increase in appetite and associated weight gain.
Patients taking ketotifen should refrain from potentially hazardous activities that involve increased alertness (for example, driving a car).
The drug can be used during lactation only if necessary, since it passes into breast milk.
Interaction
Interaction
The use of ketotifen in patients who have systematically taken corticosteroids requires a gradual reduction in previous corticosteroid therapy, as there is a risk of developing adrenal insufficiency.
Combination with oral antidiabetic agents sometimes leads to the development of thrombocytopenia.
The simultaneous use of Ketotifen and sedatives, hypnotics, antihistamines and alcohol can lead to an increase in their effect.
Overdose
Overdose
When the dose is increased, mild tremors of the limbs, anxiety and tachycardia may be observed, which disappear after the dose is reduced.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Sopharma JSC, Bulgaria
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a light-protected place, at a temperature not exceeding 25 °C |
| Manufacturer | Sofarma JSC, Bulgaria |
| Medication form | syrup |
| Brand | Sofarma JSC |
Other forms…
Related products
Buy Ketotifen Sopharma, syrup 100 ml with delivery to USA, UK, Europe and over 120 other countries.