No products in the cart.
Description
Pharmacodynamics
Antitumor drug. Doxorubicin is a cytotoxic anthracycline antibiotic isolated from Streptomyces peucetius var. caesius. The exact mechanism of doxorubicin’s antitumor action is unknown. The cytotoxic effect is thought to be due to its ability to inhibit the synthesis of DNA, RNA and proteins by introducing doxorubicin between adjacent base pairs of the DNA double helix, which prevents the helix from unfolding for subsequent replication.
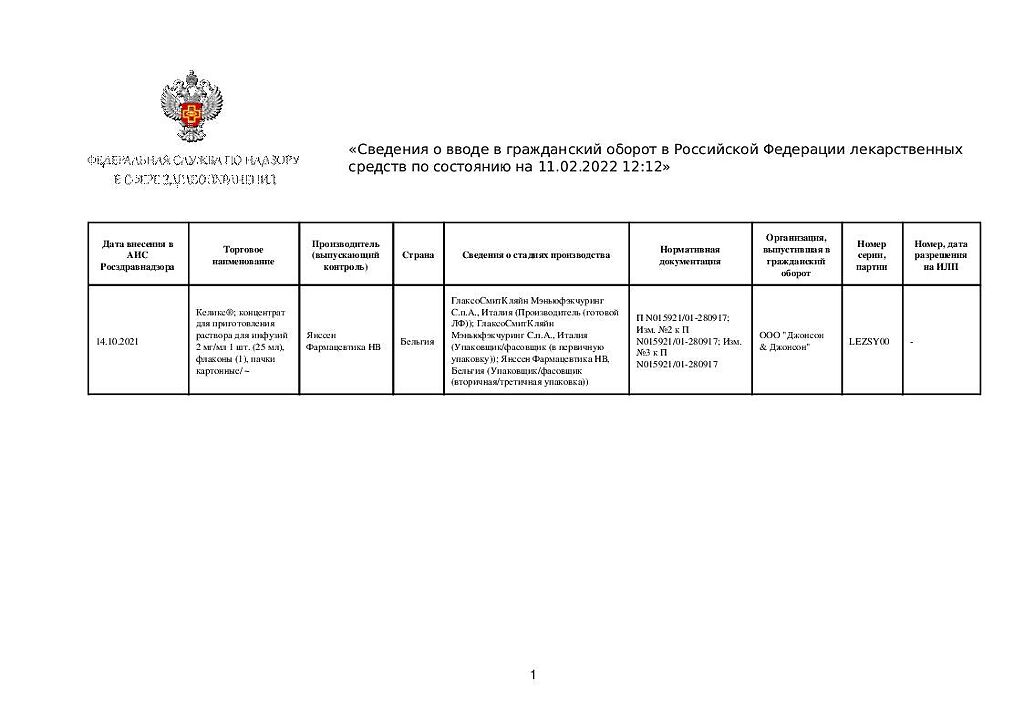
Kelix® is a pegylated liposomal form of doxorubicin that circulates long-term in the blood and provides a higher concentration of doxorubicin in tumor tissue than in normal tissue. Liposomes contain surface bound hydrophilic polymers of methoxypolyethylene glycol (MPEG). Linear groups of MPEG create a protective shell that protrudes over the liposome surface, reducing the possibility of interaction between the lipid bilayer membrane and plasma components, which protects liposomes from recognition by the phagocytic system and prolongs the circulation time of doxorubicin in the bloodstream. Pegylated liposomes also have a lipid matrix with low permeability and an internal aqueous buffer system, which in combination allows doxorubicin to be retained inside the liposome during its circulation in the bloodstream. Sufficiently small size of pegylated liposomes (average diameter of approximately 100 nm) allows them to penetrate through defects in tumor blood vessels. The results of experimental studies indicate penetration of pegylated liposomes from blood vessels and their cumulation in tumors.
Pharmacokinetics
Distribution and excretion
. When Kelix® is administered intravenously, plasma doxorubicin concentrations and AUCs are predominantly of pegylated liposomal doxorubicin (90% to 95% of measured doxorubicin, respectively), and are significantly higher than equivalent doses of conventional (unpegylated nonliposomal) doxorubicin. The pharmacokinetic profile of doxorubicin indicates that its clearance from plasma is determined by the liposomal carrier. Doxorubicin becomes available only after the liposomes leave the vascular bed and penetrate into the tissues.
Low doses (10-20 mg/m2) show linear Kelix pharmacokinetics; higher doses (20-60 mg/m2) show non-linear pharmacokinetics.
When administered at doses of 10 to 60 mg/m2, the clearance of doxorubicin averages 0.03 L/h/m2 (0.008-0.152 L/h/m2), Vd is 1.93 L/m2 (0.96-3.85 l/m2), T1/2 – 73.9 h (24-231 h).
Pharmacokinetics in special clinical cases
Pharmacokinetic parameters in liver dysfunction and hyperbilirubinemia slightly differ from pharmacokinetic parameters of normal concentration of total bilirubin.
Renal insufficiency (CK 30-156 ml/min) has no effect on pharmacokinetic parameters. There are no data on the pharmacokinetics of the drug in patients with creatinine clearance less than 30 ml/min.
Patient age (21-75 years) has no significant effect on the pharmacokinetic parameters of the drug Kelix®.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
1 ml (1 vial) contains:
The active ingredients: 2 mg (50 mg).
Auxiliary substances: sodium carbamoylmethoxypolyethyleneglycol diasteroylglycerophosphoethanolamine, phosphatidylcholine, cholesterol (cholesterol), ammonium sulfate, sucrose, histidine, hydrochloric acid, sodium hydroxide, water d/i.
In a bottle of 25 ml. One bottle is packed in a carton.
How to take, the dosage
How to take, the dosage
The drug is administered by IV drip. The drug should not be administered by jetting or undiluted.
The treatment is continued until signs of progression or unacceptable toxicity develop.
In breast and ovarian cancer, the drug is administered at a dose of 50 mg/m2 once every 4 weeks.
In the treatment of multiple myeloma, Kelix® is administered at a dose of 30 mg/m2 on day 4 of a 3-week cycle in combination with bortezomib (1.3 mg/m2 on days 1, 4, 8 and 11). Kelix is administered immediately after bortezomib. If Kelix® and bortezomib cannot be administered on day 4 of the cycle, their administration can be delayed for 48 hours. If bortezomib was administered later than the time specified in the therapy regimen, the subsequent administration of bortezomib should not be delayed until 72 h after the last dose.
If the calculated dose is less than 90 mg, the concentrate is diluted in 250 ml of 5% dextrose solution for infusion; if the dose is 90 mg or more, in 500 ml.
The first infusion is carried out at a rate not exceeding 1 mg/min in order to reduce the risk of infusion reactions. If there are no reactions, subsequent infusions can be given within 60 minutes.
Injection of the drug in patients who have had infusion reactions to the previous administration should be modified as follows: 5% of the calculated dose is administered slowly over 15 minutes. If there are no reactions, the infusion is continued with a doubled rate for another 15 minutes. If tolerated well, the infusion is continued for the next hour (total infusion time – 90 min).
The intravenous catheter and IV line should be flushed with 5% dextrose solution between injections of bortezomib and Kelix.
Modification of the dosing regimen
Tables 1-4 provide guidelines for modifying the dosing regimen of Kelix®.
The toxicity levels listed in the tables are based on the National Cancer Institute (NCI-CTC) toxicity scale.
Table 1. Dosing regimen modifications due to the development of palmar-todermal syndrome
Degree of toxicity after previous administration
of Kelix®
Dose adjustment of Kelix
sup>®
Grade I (moderate erythema, edema, or desquamation not affecting daily activities)
Inject the drug at the original dose, except in those patients who have previously experienced grade 3 to 4 toxicity. In the latter case, defer treatment for 2 weeks. Resume therapy at a 25% reduced dose, respecting the original interval between doses.
Grade II (erythema, desquamation, edema affecting but not limiting daily physical activity; small blisters or ulcerations (
Delay treatment for 2 weeks or until intensity of toxicity is reduced to grade 0-1. In those patients who have not previously experienced grade 3 or 4 toxicity, treatment may be continued at the original dose and regimen. If patients have previously experienced grade 3-4 toxicity, therapy should be resumed at a 25% reduced dose, respecting the original interval between infusions.
If no reduction in toxicity is noted after 2 weeks, treatment with Kelix should be discontinued.
Grade III (blisters, ulceration, swelling that interferes with walking or daily activities, patient cannot wear normal clothing and shoes)
/p>
Delay treatment for 2 weeks or until intensity of toxicity is reduced to grade 0-1. Resume therapy at a 25% reduced dose, respecting the original interval between injections.
If no reduction in toxicity is noted after 2 weeks, treatment with Kelix should be discontinued.
Grade IV (diffuse or localized processes leading to infectious complications, bed rest or hospitalization)
Grade IV (diffuse or localized processes leading to infectious complications, bed rest or hospitalization)/p>
Delay treatment for 2 weeks or until intensity of toxicity is reduced to grade 0-1.
Resume therapy at a 25% reduced dose, respecting the original interval between injections.
If no reduction in toxicity is noted after 2 weeks, treatment with Kelix should be discontinued.
Table 2. Dosing regimen modification due to development of stomatitis
Degree of toxicity after previous administration
of Kelix®
Dose adjustment of Kelix
sup>®
Grade I (painless ulcers, erythema or mildly painful)
Inject the drug at the original dose, except in patients who have previously had grade 3 to 4 toxicity. In the latter case, defer treatment for 2 weeks. Resume therapy at a 25% reduced dose, respecting the original interval between doses.
Grade II (painful erythema, edema, or ulcers, but patient can eat)
Grade II (painful erythema, edema, or ulcers, but patient can eat)./p>
Delay treatment for 2 weeks or until intensity of toxicity is reduced to grade 0-1. In those patients who have not previously experienced grade 3 or 4 toxicity, treatment may be continued at the original dose and regimen. If patients have previously experienced grade 3-4 toxicity, therapy should be resumed at a dose reduced by 25%, respecting the original interval between infusions. If no reduction in toxicity is noted after 2 weeks, treatment with Kelix should be discontinued.
Grade III (painful erythema, edema or ulcers, patient unable to eat)
Grade III (painful erythema, edema or ulcers, patient unable to eat)./p>
Delay treatment for 2 weeks or until intensity of toxicity is reduced to grade 0-1.
Resume therapy at a 25% reduced dose, respecting the original interval between injections.
If no reduction in toxicity is noted after 2 weeks, treatment with Kelix should be discontinued.
Grade IV (condition requires parenteral or enteral feeding)
/p>
Delay treatment for 2 weeks or until intensity of toxicity is reduced to grade 0-1.
Resume therapy at a 25% reduced dose, respecting the original interval between injections.
If no reduction in toxicity is noted after 2 weeks, treatment with Kelix should be discontinued.
Table 3. Dosing regimen modifications due to the development of hematologic toxicity
Neutrophils (in 1 µl)
Platelets (in 1 µl)
Changing dosing regimen
Grade 1
1500-1900
75,000-150,000
continued therapy without dose reduction.
Grade 2
1,000-
50,000-
If the neutrophil count is restored to 1,500 or more and the platelet count to 75,000 or more, continue treatment without reducing the dose.
Grade 3
500-
25,000-
If the neutrophil count is restored to 1,500 or more and the platelet count to 75,000 or more, continue treatment without reducing the dose.
Grade 4
Less than 500
Less than 25,000
When the neutrophil count is restored to 1,500 or more and the platelet count to 75,000 or more, continue treatment by reducing the dose by 25%, or continue treatment at the same dose with the simultaneous addition of cytokines.
Table 4. Modification of dosing regimen in multiple myeloma
Patient status
Kelix®
Fever > 38°C and neutrophil count < 1000/µl
Do not administer the drug in this cycle if an adverse reaction occurs before day 4. If it occurs after day 4, reduce the next dose by 25%.
On any day of drug administration after day 1 of each cycle:
Platelet count < 25,000 µl
Hemoglobin < 8 g/dl
Neutrophil count < 500 µl
Do not administer the drug in this cycle if an adverse reaction occurs before day 4. If it occurs after day 4, reduce the next dose by 25% if the dose of bortezomib is reduced due to hematologic toxicity.*
Grade 3-4 non-hematologic drug toxicity
Do not administer the drug until toxicity decreases to
Neuropathic pain or peripheral neuropathy
Dose adjustment is not required
* – For more information, see the instructions for bortezomib.
If a patient with multiple myeloma receiving combination therapy with Kelix® and bortezomib develops palmar-toderm syndrome or stomatitis, the dose of Kelix® should be adjusted as described in Tables 1 and 2.
In patients with impaired liver function, if serum bilirubin levels are between 1.2 and 3 mg/dL, the calculated dose is reduced by 25%. If bilirubin content exceeds 3 mg/dL, the calculated dose is reduced by 50%. If the patient tolerated the dose well (without hyperbilirubinemia or increased liver serum enzyme activity), the next dose is increased to the previous level (i.e., if the dose is reduced by 25%, it is increased to the full dose; if the dose is reduced by 50%, it is increased to 75% of the full dose). If tolerated well, the dose may be increased to the full dose in subsequent cycles. Kelix® may be administered to patients with liver metastases with concomitant hyperbilirubinemia and elevated liver enzyme activity up to 4 times the GGN. A clinical and laboratory examination of liver function, including determination of ALT, AST, ALP, and bilirubin activity, should be performed prior to administration of Kelix.
Patients with impaired renal function do not require dosing adjustments.
Interaction
Interaction
When co-administering Kelix® with cyclophosphamide or taxanes in patients with solid tumors (including ovarian and breast cancer) no increase in toxicity was found. Nevertheless, it should be taken into account that Kelix®, like other doxorubicin hydrochloride preparations, may increase the toxic effects of other antitumor drugs.
Hepatotoxic drugs, by impairing liver function, may increase doxorubicin toxicity.
The concomitant administration of live viral vaccines may intensify the replication of the vaccine virus, increase its adverse/ adverse effects and/or decrease antibody production in the patient in response to vaccine administration, so the interval between discontinuation of the drug and vaccination varies from 3 months to 1 year.
Pharmaceutical interactions
Kelix® should not be mixed with solutions other than 5% dextrose solution for infusion.
The presence of bacteriostatic additives such as benzyl alcohol in the infusion solution may cause Kelix® precipitation.
Special Instructions
Special Instructions
Kelix® should be used under the supervision of a physician experienced in cytostatic therapy.
Because Kelix® has special pharmacokinetic properties, alternating cycles of Kelix® and conventional doxorubicin therapy should not be performed.
An increased incidence of congestive heart failure is possible with a total dose of doxorubicin greater than 450 mg/m2 or at a lower dose if cardiovascular risk factors are present (prior radiotherapy to the mediastinum or therapy in combination with cyclophosphamide).
All patients receiving Kelix therapy are recommended to have ECG monitoring. Transient ECG changes, such as flattening of the T wave, decreased ST segment and clinically insignificant rhythm disturbances are not mandatory indications for withdrawal of Kelix. A specific manifestation of cardiotoxic action is a decrease in QRS complex voltages. If this change occurs, consideration should be given to myocardial biopsy as the most specific test for diagnosis of anthracycline-induced damage to the heart muscle.
Left ventricular ejection fraction measurement methods, echoCG and the preferred MUGA scan (multi-input arteriography), are among the more specific methods of monitoring cardiac function compared to ECG. These are the methods that should be used before and during the initiation of Kelix therapy. Measurement of left ventricular ejection fraction is mandatory with each subsequent administration of Kelix for total doses exceeding 450 mg/m2.
If cardiomyopathy is suspected, i.e., if left ventricular ejection fraction is lower than before treatment and/or if there is a prognostically significant decrease in this index (less than 45%), a myocardial biopsy should be performed.
The following sequencing of these tests is recommended to detect possible adverse cardiac effects of anthracycline therapy: ECG control, left ventricular ejection fraction measurement, and myocardial biopsy.
If the results of the examination suggest myocardial damage associated with Kelix therapy, a careful assessment of the ratio of the anticipated benefit of continued Kelix use to the risk of cardiotoxicity should be performed.
Congestive heart failure due to cardiomyopathy may develop unexpectedly, without previous ECG changes, and may also occur several weeks after discontinuation of therapy.
Patients with cardiac disease requiring appropriate therapy may only be prescribed Kelix if the benefit of the drug outweighs the risk to the patient.
The calculation of the cumulative dose of doxorubicin should take into account any prior or concomitant administration of cardiotoxic drugs (other anthracyclines/anthraquinones or 5-fluorouracil).
During therapy with Kelix, the peripheral blood count should be monitored regularly and, at a minimum, before each administration of the drug, with mandatory cell counts.
Persistent severe myelosuppression may lead to superinfection or bleeding.
In patients who received combined chemotherapy including doxorubicin (as with other DNA-binding antitumor drugs) there have been cases of secondary acute myeloblastic leukemia and myelodysplastic syndrome, therefore periodic hematologic monitoring is recommended for these patients.
Infusion reactions usually occur during the first infusion. Temporary suspension of the infusion usually results in resolution of symptoms without additional treatment. However, medications designed to manage infusion reactions (epinephrine, antihistamines, GCS, and other emergency medications) should be prepared when administering Kelix.
In case of symptoms of an infusion reaction, the infusion should be stopped immediately and symptomatic therapy (including antihistamines and/or GCS short-acting) should be carried out. Resumption of infusions is possible after complete resolution of all symptoms with a decrease in the rate of administration of Kelix. Infusion reactions rarely recur after the first cycle of therapy with Kelix.
Pelvicodermal syndrome usually appears after 2-3 cycles of therapy. In most patients, symptoms go away after 1-2 weeks (with or without use of GCS).
Pyridoxine at a dose of 50-150 mg/day is used to prevent and treat this syndrome. Other treatments and prophylaxis of palm-tooth syndrome are started 4-7 days after administration of Kelix. To prevent this syndrome, hands and feet should be kept cool (hand and foot baths and body baths with reduced water temperature, swimming in cool water); excessive exposure to warm/hot water should also be avoided and care should be taken not to wear tight-fitting socks, gloves, tight shoes so as not to interfere with blood circulation in the feet and hands.
The manifestations of this complication can be greatly reduced by increasing the interval between injections by 1-2 weeks or by reducing the dose. However, these reactions can be severe and may require discontinuation of therapy.
In case of symptoms of extravasal ingestion of the drug (burning, redness), immediately stop the infusion and put ice on the place of injection for 30 minutes. The drug is continued in another vein.
Contraindications
Contraindications
With caution: the drug should be used in circulatory failure, prior use of other anthracyclines, co-administration with drugs that have cytotoxic (especially myelotoxic) effects, gout (including anamnesis.In the anamnesis, urate nephrolithiasis (including anamnesis), heart disease (cardiotoxic effect may be observed at lower total doses), hepatic insufficiency.
Inhibition of medullary hematopoiesis (including Bone marrow infiltration by tumor cells, prior chemo- or radiation therapy), parasitic and infectious diseases of viral, fungal or bacterial nature (current or recent, including recent exposure to: herpes simplex, herpes zoster/viremic phase, varicella, measles, amebiasis, strongyloidiasis /established or suspected/) increase the risk for severe generalized disease.
Side effects
Side effects
Determining the frequency of side effects:
Severe adverse reactions (serious and life-threatening) account for 0 to 5% of adverse events of each type, and only in some cases do they have higher rates (leukopenia, 6.8%; stomatitis, 7.4%; neutropenia, 8.5%; palmar-subcutaneous syndrome, 19%).
Hematopoietic system: myelosuppression (anemia, thrombocytopenia, leukopenia, neutropenic fever); rarely – neutropenic fever. In patients with ovarian cancer often – hematological toxicity. In patients with breast cancer often – anemia; infrequently – leukopenia, neutropenia, thrombocytopenia. In patients with multiple myeloma (who received Kelix® in combination with bortezomib) infrequent – lymphopenia and neutropenic fever.
Cardiovascular system: infrequent – chest pain, peripheral edema, tachycardia, hot flashes, cardiotoxic effect (clinically significant change of left ventricular ejection fraction, rarely associated with symptoms of congestive heart failure); rarely – decrease of BP. In patients with multiple myeloma, rarely – decrease or increase in BP, orthostatic hypotension, phlebitis. The frequency and severity of symptoms of cardiotoxicity during therapy with Kelix is lower than during therapy – in comparable doses – with conventional doxorubicin.
Digestive system disorders: Frequent – abdominal pain, anorexia, constipation, diarrhea, mucositis, stomatitis, nausea, vomiting; infrequent – dehydration, dry mouth, dyspepsia, dysphagia, esophagitis, gastritis, gingivitis, ulceration of oral mucosa, perversion of taste; rare – flatulence, pain in the mouth. In patients with multiple myeloma, dyspepsia, aphthous stomatitis and decreased appetite, perversion of taste; infrequently, upper abdominal pain.
Dermatological reactions: Frequent – palmar-todermal syndrome (erythrodysesthesia), alopecia, skin rash, erythema; infrequent – dry skin, dermatitis, exfoliative dermatitis, maculopapular rash, vesicular bullous rash, nail lesions, skin pigmentation disorders, skin ulcers, skin itching; rarely – acne, bullous rash, epidermal desquamation, urticaria; very rarely – erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis. In patients with multiple myeloma – often – dry skin; infrequently – alopecia, allergic dermatitis, drug-induced dermatitis, erythema, papular rash, petechiae and skin itching.
Nervous system disorders: frequently – paresthesias, fatigue; infrequently – dizziness, somnolence, anxiety; rarely – neuropathy, depression, increased smooth muscle tone. In patients with multiple myeloma, headache, dizziness, insomnia, neuralgia, neuropathy, peripheral neuropathy, peripheral sensory neuropathy, and polyneuropathy; infrequently, syncope, dysesthesia, hypoesthesia, and somnolence.
Muscular system: infrequent – myalgia; rarely – ossalgia. In patients with multiple myeloma often – pain in extremities, infrequently – arthralgia, muscle weakness, muscle cramps and pain in the chest.
An organ of vision: infrequent – conjunctivitis; rarely – lacrimation.
Reproductive system and mammary glands: infrequent erythema of the scrotum.
Infusion reactions: (mainly observed during the first infusion and as a result of interactions with other drugs) anaphylactoid reactions, choking attack, facial edema, vasodilation, increased or decreased BP, urticaria, Back pain, chest pain, chills, fever, tachycardia, dyspepsia, nausea, dizziness, difficulty breathing, pharyngitis, skin rash, skin itching, increased sweating, seizures (very rare), and injection site reactions. In patients with multiple myeloma who received Kelix® and bortezomib, the incidence of infusion reactions was 3%. Temporarily stopping the infusion usually results in the resolution of these reactions without additional treatment. On subsequent administrations of Kelix, infusion reactions rarely recur.
Local reactions: if the drug gets under the skin rarely – necrosis of the surrounding tissues.
Clinically significant changes in laboratory data: infrequent – increased ACT and total bilirubin activity in blood serum, increased serum creatinine concentration; rarely – increased ALT activity in blood serum. In patients with multiple myeloma infrequent – increase of serum ALT activity, hyperkalemia, hypocalcemia, hypokalemia, hypomagnesemia, hyponatremia.
Others: Frequent – asthenia, fever, pharyngitis, increased fatigue, weakness; infrequent – headache, malaise, increased cough, shingles, oral mucosal candidiasis, increased sweating, urinary tract infection, thrombophlebitis, venous thrombosis; rarely – anaphylactic reactions, cachexia, folliculitis, herpes simplex, sepsis, upper respiratory tract infection, pulmonary embolism, dysuria, vaginitis, genital candidiasis. In patients with multiple myeloma, dyspnea, herpes simplex, shingles, weight loss, and infrequently, chills, pneumonia, upper respiratory tract infections, nasopharyngitis, elevated body temperature, viral infections, peripheral edema, nasal bleeding, and exercise dyspnea.
Combination therapy
Hematopoietic system disorders: In patients who received both combination therapy with Kelix® and bortezomib and bortezomib monotherapy, the most common were neutropenia, thrombocytopenia and anemia. The frequency of grade 3-4 neutropenia on combination therapy was higher than on bortezomib monotherapy (28% and 14%, respectively). The frequency of grade 3-4 thrombocytopenia was also higher in patients receiving combination therapy (22% and 14%, respectively). The frequency of anemia was comparable in the two groups (7% and 5%).
In the digestive system: stomatitis occurred more frequently with combination therapy (16%) than with monotherapy (3%). Most cases of stomatitis were ⤠grade 2 severity. Grade 3 stomatitis developed in 2% of patients receiving combination therapy. No cases of grade 4 stomatitis were reported. Nausea and vomiting occurred more frequently in patients receiving combination therapy (40% and 28%, respectively) than in patients receiving monotherapy (32% and 15%). Most cases were 1-2 degrees of severity.
The treatment with bortezomib or a combination of drugs (bortezomib and Kelix®) was discontinued because of adverse events in 38% of patients. The main adverse reactions that served as reasons for discontinuation of bortezomib or Kelix® were palpebral syndrome, neuralgia, peripheral neuropathy, thrombocytopenia, decreased left ventricular ejection fraction, and fatigue.
Overdose
Overdose
Symptoms: severe myelosuppression (mainly leukopenia and thrombocytopenia), toxic effects on the gastrointestinal tract (mucositis).
Treatment: in acute overdose in patients with severe myelosuppression treatment should be conducted in a hospital and include antibiotics, granulocyte and platelet transfusion and symptomatic therapy for mucositis.
Pregnancy use
Pregnancy use
The drug is contraindicated in pregnancy and during breastfeeding.
Men and women of childbearing age should use reliable contraception during treatment and for 6 months after its withdrawal.
Additional information
| Shelf life | 20 months |
|---|---|
| Conditions of storage | At 2-8 °C (do not freeze) |
| Manufacturer | TTI Biopharm Company Limited, Taiwan |
| Medication form | solution concentrate |
| Brand | TTI Biopharm Company Limited |
Related products
Buy Kelix, concentrate 2 mg/ml 25 ml with delivery to USA, UK, Europe and over 120 other countries.