No products in the cart.
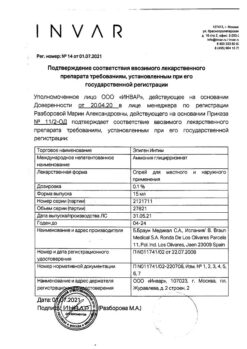
Juno Bio-T Ag Type-2 ring-shaped intrauterine contraceptive coil
€12.54 €10.45
EAN: 4811379000081
SKU: 274532
Categories: Contraceptive, Gynecology and Obstetrics, Medicine, Spiral
Description
The Juno Bio-T Ag type #2 has a silver core that increases the duration of contraceptive use.
The core of the intrauterine contraceptive is made of plastic in the form of a closed ring with a diameter of 18 mm, inside which the rod is placed.
The rod carries a bimetallic copper-silver wire with a nominal active surface area of 254 mm2 in a ring-shaped IUD “Juno Bio-T” type №2 (copper purity of at least 99.98%, silver content of at least 9.3%) and a monofilament thread is attached for control of contraceptive placement and withdrawal.
The X-ray and ultrasound contrast is ensured by the presence of bimetallic copper-silver wire on the rod. IUD insertion technique – “withdrawal” method, the diameter of the graded guide tube is 3.9mm.
The duration of contraception – not more than 7 years for IUD of circular form “Juno Bio-T Ag” type №2
Sterilization – radiation.
Indications
Indications
Contraception.
Pharmacological effect
Pharmacological effect
In the Juno Bio – T Ag model, type No. 2, the silver core increases the duration of use of contraceptives.
The anchor of the intrauterine contraceptive is made of plastic in the form of a closed ring with a diameter of 18 mm, inside of which a rod is placed.
A bimetallic copper-silver wire with a nominal active surface area of 254 mm2 is wound on the rod in a ring-shaped IUD “Junona Bio-T” type No. 2 (copper purity is at least 99.98%, silver content is at least 9.3%) and a monofilament thread is attached to control the location and removal of the contraceptive.
X-ray and ultrasound – contrast is provided by the presence of a bimetallic copper-silver wire on the rod. The IUD insertion method is the “withdrawal” method, the diameter of the graduated guide tube is 3.9 mm.
Duration of contraception – no more than 7 years for the ring-shaped IUD “Junona Bio-T Ag” type No. 2
Sterilization – radiation.
Contraindications
Contraindications
Inflammatory diseases in the genital area.
Malignant neoplasms.
Myoma and fibromyoma, deforming the uterine cavity.
Developmental anomalies.
Side Effects
Side Effects
Increased menstrual pain and discharge.
Development of inflammatory processes in the endometrium.
Perforation of the uterine wall is the most rare complication (1:4500-1:5500).
If the intrauterine device is partially located in the myometrium (first degree), it is removed vaginally.
When completely immersed in the myometrium or entering the abdominal cavity (second and third degrees), the abdominal route is used.
Manufacturer
Manufacturer
Simurg, Russia
Additional information
| Manufacturer | Simurgh, Russia |
|---|---|
| Brand | Simurgh |
Other forms…
Related products
Gynecology and Obstetrics
Gynecology and Obstetrics
Buy Juno Bio-T Ag Type-2 ring-shaped intrauterine contraceptive coil with delivery to USA, UK, Europe and over 120 other countries.