No products in the cart.
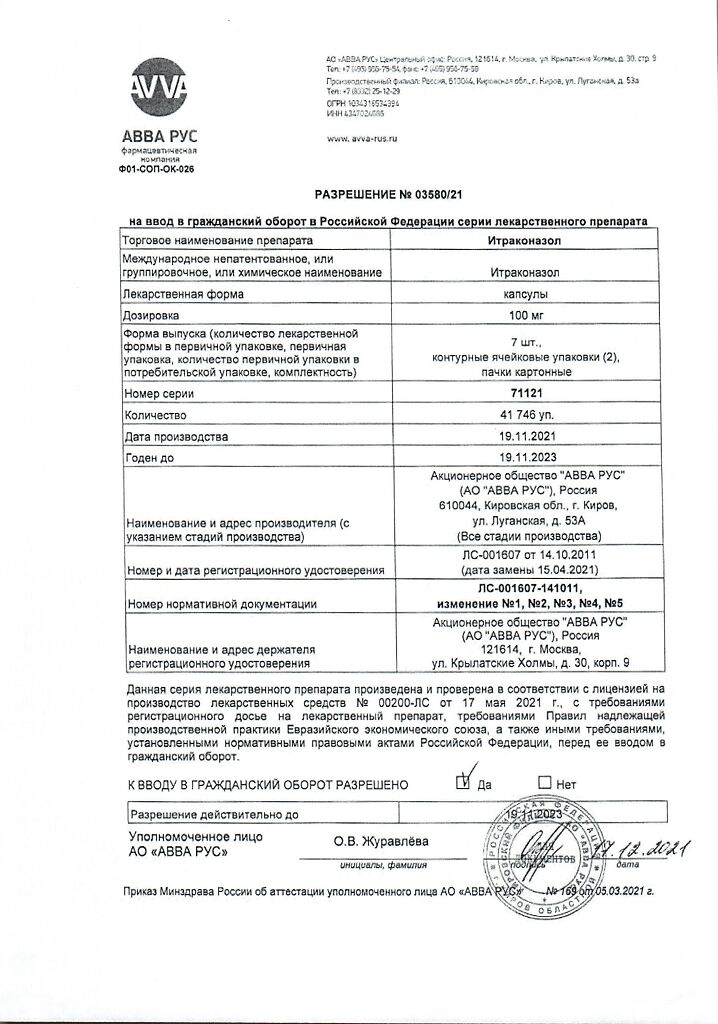
Itraconazole, 100 mg capsules 14 pcs
€13.70 €11.42
Description
Vulvovaginal candidiasis;
Indications
Indications
Vulvovaginal candidiasis;
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group:
Special instructions
Special instructions
Women of childbearing potential taking Itraconazole should use reliable methods of contraception throughout the course of treatment until the first menstrual period after its completion.
Itraconazole has been found to have a negative inotropic effect. Caution should be used when taking itraconazole and calcium channel blockers, which may have the same effect, concomitantly. Cases of chronic heart failure associated with Itraconazole have been reported.
Itraconazole should not be used in patients with chronic heart failure or a history of this disease unless the potential benefit significantly outweighs the potential risk. When individually assessing the balance of benefit and risk, factors such as the severity of the indications, dosage regimen and individual risk factors for chronic heart failure should be taken into account.
Risk factors include the presence of heart disease, such as coronary heart disease or valvular disease; serious lung diseases such as obstructive pulmonary disease; renal failure or other diseases accompanied by edema. Such patients should be informed of the signs and symptoms of congestive heart failure. Treatment should be carried out with caution, and the patient should be monitored for symptoms of congestive heart failure. If they appear, you should stop taking Itraconazole.
With reduced gastric acidity: in this condition, the absorption of itraconazole from capsules is impaired. For patients taking antacid medications (for example, aluminum hydroxide), it is recommended that they be used no earlier than 2 hours after taking Itraconazole capsules. Patients with achlorhydria or those using H2-blockers and proton pump inhibitors are advised to take Itraconazole capsules with cola drinks.
In very rare cases, severe toxic liver damage has developed with the use of Itraconazole, including cases of acute liver failure with a fatal outcome. In most cases, this was observed in patients who already had liver disease, in patients with other serious diseases receiving itraconazole therapy for systemic indications, as well as in patients receiving other drugs that have hepatotoxic effects.
Some patients had no obvious risk factors for liver damage. Several such cases occurred in the first month of therapy, and some in the first week of treatment. In this regard, it is recommended to regularly monitor liver function in patients receiving itraconazole therapy. Patients should be advised to contact their physician immediately if symptoms suggestive of hepatitis occur, such as anorexia, nausea, vomiting, weakness, abdominal pain, and dark urine.
If such symptoms occur, you should immediately stop therapy and conduct a liver function test. Patients with elevated liver enzymes or active liver disease, or history of liver toxicity while taking other drugs, should not be treated with Itraconazole unless the expected benefit justifies the risk of liver damage. In these cases, it is necessary to monitor the concentration of liver enzymes during treatment.
Liver dysfunction: Itraconazole is metabolized primarily in the liver. Since in patients with impaired liver function, the total half-life of itraconazole is slightly increased, it is recommended to monitor the plasma concentration of itraconazole and, if necessary, adjust the dose of the drug.
Renal impairment: Since the total half-life of itraconazole is slightly prolonged in patients with renal impairment, it is recommended to monitor plasma concentrations of itraconazole and adjust the dose if necessary.
Immunocompromised patients: The oral bioavailability of itraconazole may be reduced in some immunocompromised patients, such as neutropenic patients, patients with AIDS, or those undergoing organ transplantation.
Patients with life-threatening systemic fungal infections: Due to its pharmacokinetic characteristics, Itraconazole capsules are not recommended for initial treatment of life-threatening systemic mycoses in patients.
AIDS patients.
The treating physician should evaluate the need for maintenance therapy in patients with AIDS who have previously been treated for systemic fungal infections, such as sporotrichosis, blastomycosis, histoplasmosis, or cryptococcosis (both meningeal and nonmeningeal), and who are at risk of relapse.
Clinical data on the use of Itraconazole capsules in pediatric practice are limited. Itraconazole capsules should not be prescribed to children unless the expected benefit outweighs the possible risk.
Treatment should be discontinued if peripheral neuropathy occurs, which may be associated with the use of itraconazole capsules.
There is no evidence of cross-hypersensitivity to itraconazole and other azole antifungals.
Impact on the ability to drive a car and operate machinery
Itraconazole may cause dizziness and other side effects that may affect your ability to operate vehicles or other sensitive equipment.
Active ingredient
Active ingredient
Itraconazole
Composition
Composition
Each capsule contains itraconazole pellets (22%) – 0.460 g.
Pellet composition:
active substance:
Contraindications
Contraindications
Hypersensitivity, chronic heart failure, incl. history (except for treatment of life-threatening conditions); simultaneous use of CYP3A4 isoenzyme substrates that prolong the QT interval (astemizole, bepridil, cisapride, dofetilide, levacetylmethadol, mizolastatin, pimozide, quinidine, sertindole, terfenadine); HMG-CoA reductase inhibitors metabolized by the CYP3A4 isoenzyme (lovastatin, simvastatin); simultaneous oral administration of triazolam and midazolam, ergot alkaloids (dihydroergotamine, ergometrine, ergotamine, methylergotamine), nisoldipine, eletriptan; pregnancy, lactation period.
With caution
Renal and liver failure, peripheral neuropathy, risk factors: chronic heart failure (coronary heart disease, damage to the heart valves, severe lung diseases, including chronic obstructive pulmonary disease, conditions accompanied by edematous syndrome), hearing impairment, concomitant use of slow calcium channel blockers, childhood and old age.
Side Effects
Side Effects
– From the gastrointestinal tract: dyspepsia (nausea, vomiting, diarrhea, constipation, loss of appetite), abdominal pain.
Interaction
Interaction
Medicines that affect the absorption of itraconazole Medicines that reduce gastric acidity reduce the absorption of itraconazole, which is associated with the solubility of the capsule shells.
Medicines that affect the metabolism of itraconazole. Itraconazole is mainly metabolized by the CYP3A4 isoenzyme. The interaction of itraconazole with rifampicin, rifabutin and phenytoin, which are powerful inducers of the CYP3A4 isoenzyme, was studied. The study found that in these cases, the bioavailability of itraconazole and hydroxyitraconazole is significantly reduced, which leads to a significant decrease in the effectiveness of the drug. The simultaneous use of itraconazole with these drugs, which are potential inducers of microsomal liver enzymes, is not recommended. Interaction studies with other inducers of liver microsomal enzymes, such as carbamazepine, phenobarbital and isoniazid, have not been conducted, however, similar results can be expected. Potent CYP3A4 inhibitors such as ritonavir, indinavir, clarithromycin and erythromycin may increase the bioavailability of itraconazole.
Effect of itraconazole on the metabolism of other drugs. Itraconazole may inhibit the metabolism of drugs metabolized by CYP3A4. The result of this may be an increase or prolongation of their action, including side effects. Before starting to take concomitant medications, you should consult with your doctor about the metabolic pathways of this drug indicated in the instructions for medical use. After discontinuation of treatment, plasma concentrations of itraconazole decrease gradually depending on the dose and duration of treatment (see Pharmacokinetics section). This must be taken into account when discussing the migratory effect of itraconazole on concomitant medications.
Examples of such drugs are:
Drugs that should not be given concomitantly with itraconazole:
Terfenadine, astemizole, mizolastine, cisapride, dofetilide, quinidine, pimozide, levacetylmethadol, sertindole – the combined use of these drugs with itraconazole may cause increased plasma concentrations of these substances and increase the risk of QT prolongation and, in rare cases, torsade de pointes.
HMG-CoA reductase inhibitors metabolized by the CYP3A4 isoenzyme, such as simvastatin and lovastatin,
midazolam for oral administration and triazolam,
ergot alkaloids such as dihydroergotamine, ergometrine, ergotamine and methylergometrine,
Blockers of “slow” calcium channels – in addition to the possible pharmacokinetic interaction associated with the common metabolic pathway involving the isoenzyme CYP3A4, blockers of “slow” calcium channels may have a negative inotropic effect, which is enhanced when taken simultaneously with itraconazole.
Drugs, when prescribed, it is necessary to monitor their concentration in plasma, action, and side effects. When co-administered with itraconazole, the dose of these drugs should be reduced, if necessary.
Indirect anticoagulants;
HIV protease inhibitors such as ritonavir, indinavir, saquinavir;
Some anticancer drugs such as rose vinca alkaloids, busulfan, docetaxel, trimetrexate;
“slow” calcium channel blockers metabolized by the CYP3A4 isoenzyme, such as verapamil and dihydropyridine derivatives;
Some immunosuppressive drugs: cyclosporine, tacrolimus, sirolimus (also known as rapamycin);
Some HMG-CoA reductase inhibitors metabolized by the CYP3A4 isoenzyme, such as atorvastatins;
Some glucocorticosteroids such as budesonide, dexamethasone and methylprednisolone;
Other drugs: digoxin, carbamazepine, buspirone alfentanil, alprazolam, brotizolam, intravenous midazolam, rifabutin, ebastine, reboxetine, cilostazol, disoliramide, eletriptan, halofantrine, repaglinide.
No interaction was found between itraconazole and zidovudine and fluvastatin.
There was no effect of itraconazole on the metabolism of ethinyl estradiol and norethisterone.
Effect on plasma protein binding.
In vitro studies have demonstrated that there is no interaction between itraconazole and drugs such as imipramine, propranolol, diazepam, cimetidine, indomethacin, tolbutamide and sulfamethazine when bound to plasma proteins.
Overdose
Overdose
No data available.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 ° C.
Shelf life
Shelf life
3 years.
Manufacturer
Manufacturer
ABVA RUS, Russia
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 ° C. |
| Manufacturer | Avva Rus, Russia |
| Medication form | capsules |
| Brand | Avva Rus |
Related products
Buy Itraconazole, 100 mg capsules 14 pcs with delivery to USA, UK, Europe and over 120 other countries.