No products in the cart.
Description
Raltegravir inhibits the catalytic activity of HIV integrase, an enzyme involved in HIV virus replication. Inhibition of integrase prevents covalent insertion (integration) of the HIV genome into the host cell genome in the early stages of infection. HIV genomes not incorporated into human DNA are unable to induce the production of new viral particles, so suppression of the integration process prevents the spread of viral infection in the body. The inhibitory ability of raltegravir against human phosphotransferases, including DNA polymerases α, β and γ, is expressed insignificantly.
Microbiology
At a plasma concentration of 31±20 nmol/L, raltegravir provided 95% suppression of viral replication (95% inhibitory concentration, IR95) in cell cultures of human T lymphocytes infected with cell culture-adapted variant H9IIIB HIV-1 compared with control virus-infected cell cultures. IR95 was achieved at concentrations ranging from 6 to 50 nmol/L in cultures of human mitogen-activated peripheral blood mononuclear cells infected with various primary clinical strains of HIV-1, including strains of 5 non-B HIV-1 subtypes as well as strains resistant to HIV reverse transcriptase inhibitors and protease inhibitors.
In a single infection cycle assay, raltegravir suppressed infection caused by 23 strains of HIV representing 5 non-B subtypes and 5 circulating recombinant forms, with IR50 – 5-12 nmol/L. Raltegravir also suppressed HIV-2 strain replication when tested on CEMx174 cells (IR95 = 6 nmol/L). When raltegravir and nucleoside reverse transcriptase inhibitors (zidovudine, zalcitabine, stavudine, abacavir, tenofovir, didanosine, and lamivudine) were simultaneously introduced into a culture of human T lymphocytes infected with variant H9IIIB of HIV-1 virus, non-nucleoside reverse transcriptase inhibitors (efavirenz, nevirapine and delavirdine), HIV protease inhibitors (indinavir, saquinavir, ritonavir, amprenavir, lopinavir, nelfinavir and atazanavir) or fusion inhibitor (enfuvirtide) had additive to synergistic antiretroviral activity.
Drug resistance
. HIV-1 integrase mutations that contribute to raltegravir-resistant virus strains (developed in vitro or in patients taking raltegravir) mainly involve substitutions at positions 155 (N155 substitution for H) 148 (Q148 substitution for H, K, or R) or 143 (Y143 substitution for C, H, or R), combined with one or more additional mutations (e.g., L74M, E92Q, T97A, E138A/K, G140A/S, V151I, G163R, S230R).
The recombinant viruses with a single primary mutation (Q148H, K or R, or N155H) were characterized by reduced replication ability and reduced sensitivity to raltegravir in vitro. Secondary mutations of the virus further reduced sensitivity to raltegravir, sometimes compensating for the reduced replication ability of the virus.
Mutations associated with the development of resistance to raltegravir can also lead to the formation of resistance to another integrase chain transfer inhibitor elvitegravir. The substitution at position 143 reduces the sensitivity to raltegravir to a greater extent than the sensitivity to elvitegravir, while mutations in E92Q cause greater resistance to elvitegravir than to raltegravir. Viruses with a mutation at position 148 in combination with one or more additional mutations causing resistance to raltegravir can also show clinically significant resistance to dolutegravir.
The effect on cardiac electrophysiologic activity or ECG parameters
In a placebo-controlled clinical trial involving healthy volunteers, a single administration of 1600 mg of raltegravir had no effect on QTc interval duration despite the fact that Cmax of raltegravir in plasma was 4 times greater than that of a single dose of 400 mg raltegravir.
Indications
Indications
Treatment of HIV-1 infection in combination with other antiretroviral drugs in children aged 2-11 years, both previously received and not previously received antiretroviral therapy.
Pharmacological effect
Pharmacological effect
Raltegravir inhibits the catalytic activity of HIV integrase, an enzyme involved in the replication of the HIV virus. Integrase inhibition prevents the covalent introduction (integration) of the HIV genome into the host cell genome during the early stages of infection. HIV genomes that are not included in human DNA are not capable of inducing the production of new viral particles, therefore suppression of the integration process prevents the spread of viral infection in the body. The inhibitory ability of raltegravir against human phosphotransferases, including DNA polymerases α, β and γ, is insignificant.
Microbiology
At a plasma concentration of 31 ± 20 nmol/L, raltegravir suppressed viral replication by 95% (95% inhibitory concentration, IC95) in cell cultures of human T lymphocytes infected with the cell-adapted H9IIIB variant of HIV-1 compared to a control virus-infected cell culture. IC95 was achieved at concentrations ranging from 6 to 50 nmol/L in cultures of human mitogen-activated peripheral blood mononuclear cells infected with various primary clinical strains of HIV-1, including strains of the 5 non-B subtypes of HIV-1, as well as strains resistant to reverse transcriptase inhibitors and HIV protease inhibitors.
When analyzing one cycle of infection, raltegravir suppressed infection caused by 23 strains of HIV, representing 5 non-B subtypes and 5 circulating recombinant forms, with an IC50 of 5-12 nmol/l. Raltegravir also suppressed the replication of HIV-2 strains when tested on CEMx174 cells (IC95 = 6 nmol/l). With the simultaneous introduction into culture of human T lymphocytes infected with the H9IIIB variant of the HIV-1 virus, raltegravir and nucleoside reverse transcriptase inhibitors (zidovudine, zalcitabine, stavudine, abacavir, tenofovir, didanosine and lamivudine), non-nucleoside reverse transcriptase inhibitors (efavirenz, nevirapine and delavirdine), HIV protease inhibitors (indinavir, additive to synergistic antiretroviral activity was observed.
Drug resistance
HIV-1 integrase mutations that contribute to the emergence of raltegravir-resistant virus strains (developed in vitro or in patients treated with raltegravir) mainly include substitutions at positions 155 (N155 substitution to H), 148 (Q148 substitution to H, K or R) or 143 (Y143 substitution to C, H or R), in combination with one or more additional mutations (for example, L74M, E92Q, T97A, E138A/K, G140A/S, V151I, G163R, S230R).
Recombinant viruses with one primary mutation (Q148H, K or R or N155H) were characterized by reduced replication ability and reduced sensitivity to raltegravir in vitro. Secondary mutations of the virus further reduced sensitivity to raltegravir, sometimes compensating for the reduced ability of the virus to replicate.
Mutations associated with the development of resistance to raltegravir can also lead to the development of resistance to another integrase strand transfer inhibitor, elvitegravir. Substitution at position 143 reduces sensitivity to raltegravir more than sensitivity to elvitegravir, while mutations in E92Q cause greater resistance to elvitegravir than to raltegravir. Viruses with a mutation at position 148 in combination with one or more additional mutations conferring resistance to raltegravir may also exhibit clinically significant resistance to dolutegravir.
Effect on electrophysiological activity of the heart or ECG parameters
In a placebo-controlled clinical study in healthy volunteers, a single dose of raltegravir 1600 mg had no effect on the QTc interval, although the plasma Cmax of raltegravir was 4 times greater than that observed with a single dose of raltegravir 400 mg.
Special instructions
Special instructions
Patients should be informed that current antiretroviral drugs do not cure HIV infection or prevent transmission of HIV to others through blood or sexual contact. During treatment with Isentress®, patients should continue to follow appropriate safety precautions. Patients should also be informed that they may still develop infections or other conditions common among HIV-infected patients (opportunistic infections). It is very important to remain under medical supervision during therapy with Isentress®.
Raltegravir has a relatively low genetic barrier to the development of resistance, therefore, to increase the effectiveness of treatment and reduce the risk of developing drug resistance, raltegravir should be prescribed in combination with two other active antiretroviral agents, if possible. It is important to explain to patients the need to study the instructions for use before starting therapy with Isentress® and re-read it every time they receive another prescription from a doctor. Patients should be informed to notify their physician if any unusual symptoms occur or if any known symptom persists or worsens.
Immune reconstitution syndrome
At the initial stages of combined ART, HIV-infected patients with severe immunodeficiency may develop the so-called immune reconstitution syndrome, i.e. inflammatory reaction to asymptomatic or residual opportunistic infections (including cytomegalovirus retinitis, Pneumocystis jiroveci pneumonia, disseminated or focal mycobacterial infections). This may worsen the clinical condition and intensify existing symptoms. Typically, such a reaction can be observed in the first weeks or months after starting combination therapy. Any inflammatory symptoms should be assessed and treatment prescribed if necessary.
In the development of immune reconstitution syndrome, autoimmune disorders such as Graves’ disease have been described. The development of such disorders can be observed many months after the start of treatment.
Osteonecrosis
Despite the fact that the etiology of this complication is considered multifactorial (including corticosteroid therapy, alcohol consumption, severe immunodeficiency, high body mass index), cases of the development of osteonecrosis have been described, especially in the later stages of HIV infection and/or with long-term use of combined ART. Patients who develop symptoms such as joint pain, stiffness or limited mobility need urgent consultation with a specialist.
Severe skin reactions and hypersensitivity reactions
There has been evidence of severe (potentially life-threatening) and fatal adverse skin reactions in patients taking Isentress® in combination therapy with other drugs associated with these adverse reactions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis. Hypersensitivity reactions have also been reported, manifesting as a generalized rash and sometimes organ dysfunction, including liver failure. You should immediately stop using Isentress® and other drugs suspected of causing these reactions if signs or symptoms of severe skin reactions or hypersensitivity reactions occur (including severe skin rash or rash accompanied by fever, malaise, weakness, muscle or joint pain, blistering of the skin, lesions in the mouth, conjunctivitis, facial swelling, hepatitis, eosinophilia, angioedema, but not limited only to them). In these cases, it is necessary to monitor the clinical status, including the activity of hepatic aminotransferases, and initiate appropriate therapy. Failure to promptly discontinue therapy with Isentress® or other drugs associated with these adverse reactions after the onset of severe rash may lead to life-threatening reactions.
Myopathy and rhabdomyolysis
Myopathy and rhabdomyolysis have been reported. Caution should be exercised when prescribing the drug to patients with a history of myopathy and rhabdomyolysis or any factors predisposing to their development, in particular, with concomitant therapy with drugs that can cause these adverse reactions.
Liver dysfunction
The safety and effectiveness of Isentress® in patients with severe concomitant liver diseases have not been established. Caution should be exercised when prescribing Isentress® to patients with severe liver dysfunction.
Patients with existing liver dysfunction, including chronic hepatitis, have an increased incidence of liver dysfunction with combination ART and should be monitored according to standard practice. If such patients show signs of worsening liver disease, interruption or discontinuation of treatment should be considered.
Patients with chronic hepatitis B or C who are also receiving combination ART are at risk of developing severe and potentially fatal liver adverse events.
Skin rash
In patients who have previously received ART, when using the drug Isentress® simultaneously with darunavir, skin rash is observed more often than in patients using the drugs separately (see section “Side effects”).
Depression
Depression, including suicidal ideation and behavior, has been observed primarily in patients with a history of depression or psychiatric illness. Caution should be exercised when prescribing Isentress® to patients with a history of depression or psychiatric illness.
Concomitant use with other drugs
Strong inductors UDF-GT1A1. Caution should be exercised when prescribing Isentress® concomitantly with strong UDP-GT1A1 inducers, such as rifampicin, due to the decrease in plasma concentrations of raltegravir they cause. If combination therapy with rifampicin and Isentress® is necessary, the dose of Isentress® should be increased by 2 times in adult patients. There are no data to adjust drug doses when using Isentress® and rifampicin simultaneously in patients under 18 years of age (see section “Drug Interactions”).
Antacids. The simultaneous use of Isentress® with antacids containing aluminum or magnesium leads to a decrease in the concentration of raltegravir in the blood plasma.
The simultaneous use of Isentress® with antacids containing aluminum or magnesium is not recommended (see section “Drug interactions”).
Fructose and sorbitol
The drug Isentress® in the dosage form of chewable tablets contains fructose and sorbitol. Patients with rare hereditary disorders such as fructose intolerance should not take Isentress®.
Phenylketonuria
The drug Isentress® in the dosage form of chewable tablets as part of the masking flavor contains phenylalanine as a component of aspartame. Each 25 mg chewable tablet of Isentress® contains approximately 0.05 mg phenylalanine, and each 100 mg chewable tablet contains approximately 0.10 mg phenylalanine. Phenylalanine may be harmful to patients with phenylketonuria.
Impact on the ability to drive vehicles and machinery
Studies have not been conducted to study the effect on the ability to drive vehicles and use machinery. Considering the possibility of developing dizziness, weakness, drowsiness and blurred vision during treatment with Isentress®, special care must be taken when driving and operating machinery.
Active ingredient
Active ingredient
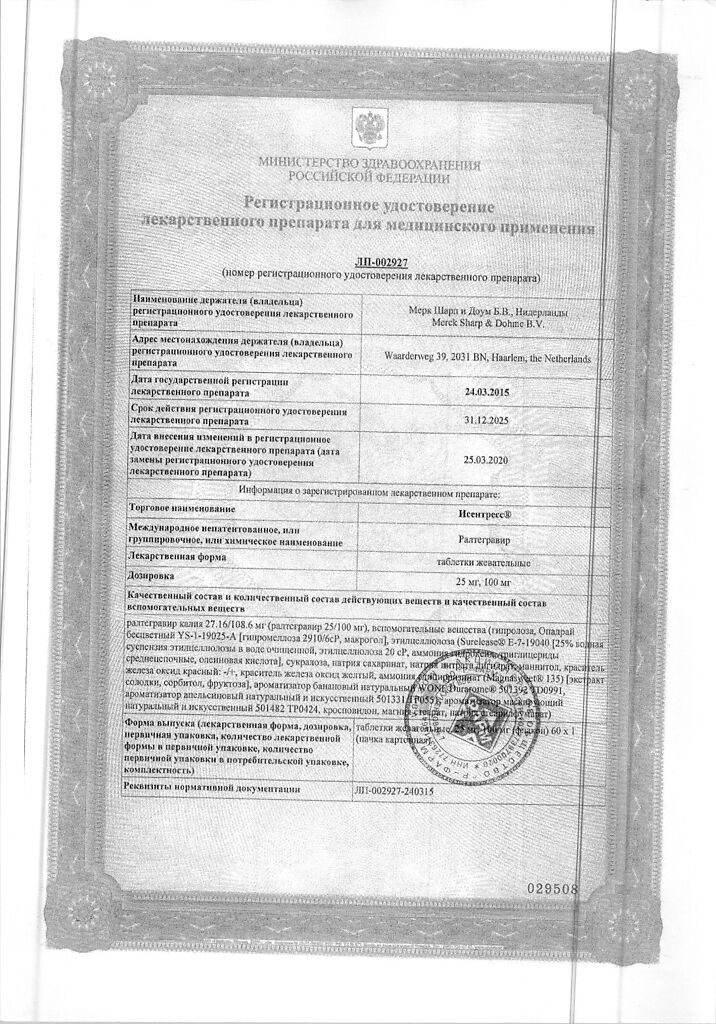
Raltegravir
Composition
Composition
1 tab. contains:
Contraindications
Contraindications
hypersensitivity to raltegravir and other components of the drug;
children under 2 years of age and body weight less than 7 kg;
pregnancy;
lactation period;
sucrase/isomaltase deficiency, fructose intolerance, glucose-galactose malabsorption;
phenylketonuria.
With caution:
myopathy and rhabdomyolysis (including a history), the presence of conditions and factors predisposing to their development;
severe liver failure;
simultaneous use with strong inducers of UDP-GT1A1 (rifampicin);
simultaneous use of the drug Isentress® with antacids containing aluminum or magnesium;
depression, including suicidal ideation and behavior (observed mainly in patients with a history of depression or psychiatric illness). Caution should be exercised when prescribing Isentress® to patients with a history of depression or psychiatric illness.
Side Effects
Side Effects
The safety profile of Isentress® is based on the results of pooled safety data obtained from clinical studies involving patients previously treated with antiretroviral therapy (ART) and patients not previously treated with ART.
In a pooled analysis of clinical trials of antiretroviral therapy in adults previously treated with ART, the rate of discontinuation of therapy due to adverse reactions was 3.9% in the group of patients taking Isentress® and optimized adjunctive therapy (OCT), and 4.6% in the group of patients taking placebo and OCT. The rate of discontinuation of therapy due to adverse reactions in adult patients who had not previously received ART was 5.0% in the group of patients taking Isentress® simultaneously with emtricitabine and tenofovir, and 10.0% in the group of patients taking efavirenz, emtricitabine and tenofovir simultaneously.
Below are data on adverse events observed in clinical studies, with varying degrees of probability associated with the drug Isentress® or its combination with other ART.
Adverse events are listed according to systemic organ classes and frequency classification: often (≥1/100 and <1/10), infrequently (≥1/1000 and <1/100).
Infectious and parasitic diseases: uncommon – genital herpes, folliculitis, gastroenteritis, herpes simplex, herpes infection, herpes zoster, influenza, lymph node abscess, molluscum contagiosum, nasopharyngitis, upper respiratory tract infection.
Benign, malignant and unspecified neoplasms (including cysts and polyps): uncommon – cutaneous papillomatosis.
From the hematopoietic and lymphatic systems: infrequently – anemia, iron deficiency anemia, painful lymph nodes, lymphadenopathy, neutropenia, thrombocytopenia1.
From the immune system: infrequently – immune reconstitution syndrome, hypersensitivity to the drug, hypersensitivity reactions.
Metabolism: often – loss of appetite; uncommon – cachexia, diabetes mellitus, dyslipidemia, hypercholesterolemia, hyperglycemia, hyperlipidemia, hyperphagia, increased appetite, polydipsia, lipid metabolism disorders.
Mental disorders: often – unusual dreams, insomnia, nightmares, behavioral disorders2, depression; uncommon – mental disorders, suicide attempts, anxiety, confusion, depressed mood, major depressive disorder, middle of the night insomnia, mood changes, panic attacks, sleep disturbances, suicidal ideation1, suicidal behavior1 (especially in patients with a history of psychiatric illnesses).
From the nervous system: often – dizziness, headache, psychomotor hyperreactivity2, infrequently – amnesia, carpal tunnel syndrome, cognitive disorders, attention disorders, postural dizziness, dysgeusia, hypersomnia, hypoesthesia, lethargy, memory disorder, migraine, peripheral neuropathy, paresthesia, drowsiness, tension headache, tremor, decreased sleep quality.
From the side of the organ of vision: infrequently – decreased visual acuity.
From the organ of hearing and labyrinthine disorders: often – vertigo; infrequent – tinnitus.
From the cardiovascular system: infrequently – palpitations, sinus bradycardia, ventricular extrasystole, flushing of the face with a feeling of heat, arterial hypertension.
From the respiratory system, chest and mediastinal organs: uncommon – dysphonia, nosebleeds, nasal congestion.
From the digestive system: often – a feeling of fullness in the abdomen, abdominal pain, diarrhea, flatulence, nausea, vomiting, dyspepsia; uncommon – gastritis, feeling of discomfort in the abdomen, pain in the upper abdomen, pain in the abdomen, discomfort in the anus, constipation, dry mouth, discomfort in the epigastric region, erosive duodenitis, belching, gastroesophageal reflux, gingivitis, glossitis, pain when swallowing, acute pancreatitis, peptic ulcer, rectal bleeding.
From the liver and biliary tract: infrequently – hepatitis, hepatic steatosis, alcoholic hepatitis, liver failure1.
From the skin and subcutaneous tissues: often – skin rash; uncommon – acne, alopecia, acne, dry skin, erythema, facial lipoatrophy, hyperhidrosis, lipoatrophy, acquired lipodystrophy, lipohypertrophy, night sweats, prurigo, itching (local and generalized), macular rash, maculopapular rash, pruritic rash, urticaria, xeroderma, other skin lesions, Stevens-Johnson syndrome1, drug rash with eosinophilia and systemic symptoms (DRESS syndrome)1.
From the musculoskeletal system: uncommon – arthralgia, arthritis, back pain, flank pain, myalgia, neck pain, osteopenia, pain in the extremities, osteoporosis, polyarthritis, tendinitis, myopathy, rhabdomyolysis1.
From the kidneys and urinary tract: uncommon – renal failure, nephritis, nephrolithiasis, nocturia, kidney cyst, renal dysfunction, tubulointerstitial nephritis.
From the genital organs and breast: uncommon – erectile dysfunction, gynecomastia, menopausal symptoms.
General disorders: often – asthenia, weakness, fever; uncommon – chest discomfort, chills, facial swelling, increased volume of adipose tissue, anxiety, malaise, submandibular neoplasm, peripheral edema, pain.
Laboratory and instrumental data: often – increased plasma activity of ALT, AST, pancreatic lipase and amylase, increased concentration of triglycerides and the number of atypical lymphocytes; infrequently – a decrease in the absolute number of plasma neutrophils; increased activity in plasma of alkaline phosphatase, CPK, decreased albumin concentration; increased concentrations of bilirubin, cholesterol, creatinine, glucose (including determined on an empty stomach), urea nitrogen, HDL cholesterol, LDL cholesterol; increase in INR value; decrease in the number of platelets and leukocytes in the blood; the presence of glucose in the urine, the presence of red blood cells in the urine; increase in waist circumference; increase or decrease in body weight.
Injuries, intoxication and complications of manipulation: uncommon – unintentional overdose.
1Adverse events not related to the use of the drug Isentress®, which were observed in the post-registration period and were not observed during clinical trials.
2 One pediatric patient experienced drug-related adverse reactions: grade 3 psychomotor hyperreactivity and behavioral disturbance; This patient also experienced insomnia.
During clinical trials in patients who had previously received and had not received ART, cases of the development of malignant neoplasms were observed when using a combination of the drug Isentress® with other antiretroviral drugs. The characteristics and incidence of malignancies were consistent with those in patients with severe immunodeficiency. The risk of developing malignant neoplasms in clinical studies was the same both in the groups of patients taking the drug Isentress® and in the groups of patients taking comparator drugs.
In patients taking the drug Isentress®, an increase in CPK activity of 2-4 degrees was observed. Cases of myopathy and rhabdomyolysis have been observed. In patients with a history of myopathy or rhabdomyolysis, as well as other risk factors (including concomitant therapy), the drug should be prescribed with caution.
Osteonecrosis has been reported, especially in patients with established risk factors, advanced HIV disease, or long-term exposure to combination ART. The frequency of its development is unknown.
In clinical studies in patients previously treated with ART, skin rash, regardless of etiology, was more often observed when using Isentress® simultaneously with darunavir than when using these drugs separately. However, the incidence of drug-related skin rash was comparable between these treatment groups. The skin rash was mild to moderate in severity and did not affect continuation of ART. In ART-naïve patients, rash development was less common when treated with Isentress® in combination with emtricitabine and tenofovir than when treated with efavirenz in combination with emtricitabine and tenofovir.
Patients with co-infection with hepatitis B and/or hepatitis C
Overall, the safety profile of Isentress® in ART-naive and ART-naïve patients co-infected with chronic (but not acute) active hepatitis B and/or hepatitis C was similar to the safety profile in patients without hepatitis B and/or hepatitis C co-infection, although the incidence of ALT and AST abnormalities was sometimes higher in groups with hepatitis B and/or hepatitis C co-infection.
Children
According to the results of clinical studies on the use of raltegravir in recommended doses in combination with other antiretroviral drugs in HIV-1 infected children and adolescents from 2 to 18 years of age, it was found that the frequency, type and severity of adverse reactions associated with taking the drug were comparable to those in adult patients. One patient experienced drug-related adverse reactions: grade 3 psychomotor hyperactivity, behavioral disturbances, and insomnia. Another patient experienced a grade 2 serious adverse reaction, allergic rash. Another patient had grade 4 AST and grade 3 ALT elevations, which were considered serious.
Interaction
Interaction
In vitro studies have shown that raltegravir is not a substrate of cytochrome P450 isoenzymes and does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP3A. In addition, in vitro, raltegravir does not induce CYP3A4 and is not an inhibitor of P-glycoprotein-mediated transport. Based on these data, we can conclude that Isentress® does not affect the pharmacokinetic parameters of drugs that are substrates of these enzymes or P-glycoprotein.
As shown by in vitro and in vivo studies, raltegravir is eliminated mainly through metabolism (glucuronidation) via the UDP-GT1A1-mediated pathway. Although in vitro studies have shown that raltgegravir is not an inhibitor of UDP-GT1A1 and 2B7, one clinical study showed some evidence of UDP-GT1A1 inhibition in vivo based on the observed effect on bilirubin glucuronidation. However, this effect is unlikely to result in a clinically significant drug interaction.
Significant inter- and intraindividual variability was observed in the pharmacokinetics of raltegravir. The drug interaction information below is based on geometric means; the effect in an individual patient cannot be accurately predicted.
Effect of raltegravir on the pharmacokinetics of other drugs
In drug interaction studies, raltegravir did not have a clinically significant effect on the pharmacokinetics of etravirine, maraviroc, tenofovir, hormonal contraceptives, methadone and midazolam.
In some studies, with simultaneous use of Isentress® with darunavir, a slight decrease in the concentration of darunavir in blood plasma was observed. The mechanism of this phenomenon is unknown. However, the effect of raltegravir on darunavir plasma concentrations is not considered to be clinically significant.
Effect of other drugs on the pharmacokinetics of raltegravir
Caution should be exercised when simultaneous use of Isentress® with strong inducers of UDP-GT1A1 (for example, rifampicin), given that raltegravir is metabolized mainly through UDP-GT1A1. Rifampicin reduces plasma concentrations of raltegravir. The effect on the effectiveness of raltegravir is unknown. However, if concomitant use with rifampicin cannot be avoided, the dose of Isentress® in adults can be doubled. There are no data on the simultaneous use of Isentress® and rifampicin in patients under 18 years of age.
The effect of other strong inducers of drug-metabolizing isoenzymes, such as phenytoin and phenobarbital, on the UDP-GT1A1 system is unknown. Less strong inducers (for example, efavirenz, nevirapine, etravirine, rifabutin, corticosteroids, St. John’s wort, pioglitazone) can be used simultaneously with Isentress® at the recommended dose.
Concomitant use of Isentress® with strong UDP-GT1A1 inhibitors (for example, atazanavir) may increase the plasma concentration of raltegravir. Less potent UDP-GT1A1 inhibitors (eg, indinavir and saquinavir) may also increase plasma concentrations of raltegravir, but to a lesser extent compared to atazanavir. In addition, tenofovir may increase plasma concentrations of raltegravir, but the mechanism of this effect is unknown.
The safety profile observed in patients treated with atazanavir and/or tenofovir is generally identical to that of patients who did not take these drugs, so no dose adjustment is required.
Concomitant use of Isentress® with antacids containing bivalent metal ions may reduce the absorption of raltegravir by chelation, which leads to a decrease in the concentration of raltegravir in the blood plasma. Because Taking antacids containing aluminum or magnesium 6 hours after taking Isentress® leads to a significant decrease in the concentration of raltegravir in the blood plasma; simultaneous use of Isentress® and antacids containing aluminum or magnesium is not recommended.
The simultaneous use of Isentress® with antacids containing calcium carbonate reduces the concentration of raltegravir in the blood plasma, but this interaction is not considered clinically significant. As a result, when using the drug Isentress® simultaneously with antacids containing calcium carbonate, no dose adjustment is required.
The simultaneous use of Isentress® with other drugs that increase the pH of gastric juice (for example, omeprazole or famotidine) may increase the rate of absorption of raltegravir and, accordingly, the concentration of raltegravir in the blood plasma. In a clinical study, the safety profile in the subgroup of patients taking proton pump inhibitors or histamine H2 receptor blockers was comparable to the safety profile in the subgroup of patients not taking these drugs. Dose adjustment of Isentress® is not required when used concomitantly with proton pump inhibitors or histamine H2 receptor blockers.
All drug interaction studies were conducted in adult patients.
Table 2. Data on pharmacokinetic drug interactions in adult patients.
Medicinal product taking into account the therapeutic area of application
Interaction (mechanism if known)
Recommendations for adjusting the dosage regimen
Antiretroviral drugs
Protease inhibitors
atazanavir/ritonavir
(raltegravir 400 mg 2 times/day)
raltegravir AUC↑ 41%
raltegravir C12h↑ 77%
raltegavir Cmax↑ 24%
(UDP-GT1A1 inhibition)
No dose adjustment of Isentress® is required.
tipranavir/ritonavir (raltegravir 400 mg 2 times a day)
raltegravir AUC↓ 24%
raltegravir C12h↓ 55%
raltegavir Cmax↓ 18%
(induction of UDP-GT1A1)
No dose adjustment of Isentress® is required.
Nonnucleoside reverse transcriptase inhibitors (NNRTIs)
efavirenz (raltegravir 400 mg once a day)
raltegravir AUC↓ 36%
raltegravir C12h↓ 21%
raltegavir Cmax↓ 36%
(induction of UDP-GT1A1)
No dose adjustment of Isentress® is required.
etravirine (raltegravir 400 mg 2 times a day)
raltegravir AUC↓ 10%
raltegravir C12h↓ 34%
raltegavir Cmax↓ 11%
(UDP-GT1A1 induction) etravirine AUC↑ 10%
etravirine C12h ↑ 17%
etravirine Cmax ↑ 4%
No dose adjustment is required for Isentress® or etravirine.
Nucleoside reverse transcriptase inhibitors (NRTIs)
tenofovir (raltegravir 400 mg 2 times a day)
raltegravir AUC↑ 49%
raltegravir C12h↑ 3%
raltegavir Cmax↑ 64%
(mechanism of interaction unknown)
tenofovir AUC ↓10%
tenofovir C12h ↓ 13%
tenofovir Cmax↓ 23%
No dose adjustment is required for Isentress® or tenofovir disoproxil fumarate.
CCR5 chemokine receptor antagonists
maraviroc (raltegravir 400 mg 2 times a day)
raltegravir AUC↓ 37%
raltegravir C12h↓28% raltegavir Cmax ↓ 33%
(mechanism of interaction unknown)
maraviroc AUC↓ 14%
maraviroc C12h ↓ 10%
maraviroc Cmax ↓ 21%
No dose adjustment is required for Isentress® or maraviroc.
Medicines against hepatitis C virus
Hepatitis C virus NS3/4A protease inhibitors
boceprevir (raltegravir 400 mg once a day)
raltegravir AUC↑ 4%
raltegravir C12h↓ 25%
raltegavir Cmax↑ 11%
(mechanism of interaction unknown)
No dose adjustment of Isentress® or boceprevir is required.
Antimicrobials
Anti-tuberculosis drugs
rifampicin (raltegravir 400 mg once a day)
raltegravir AUC↓ 40%
raltegravir C12h↓ 61%
raltegavir Cmax↓ 38%
(induction of UDP-GT1A1)
Rifampicin reduces the plasma concentration of raltegravir. If combination therapy with rifampicin cannot be avoided, it is necessary to consider increasing the dose of Isentress® by 2 times.
Sedatives
midazolam (raltegravir 400 mg 2 times a day)
midazolam AUC↓ 8%
midozolam Cmax↑ 3%
No dose adjustment of Isentress® or midazolam is required. The data obtained indicate that raltegravir is not an inducer or inhibitor of CYP3A4 and that raltegravir does not affect the pharmacokinetics of drugs that are CYP3A4 substrates.
Antacids
Antacids containing aluminum or magnesium (raltegravir 400 mg 2 times a day)
Simultaneously with raltegravir
raltegravir AUC↓ 49%
raltegravir C12h↓63% raltegavir Cmax ↓ 44%
2 hours before taking raltegravir
raltegravir AUC↓ 51%
raltegravir C12h↓56% raltegavir Cmax ↓51%
2 hours after taking raltegravir
raltegravir AUC↓ 30%
raltegravir C12h↓57% raltegavir Cmax ↓22%
6 hours before taking raltegravir
raltegravir AUC↓ 13%
raltegravir C12h↓50% raltegavir Cmax ↓10%
6 hours after taking raltegravir
raltegravir AUC↓ 11%
raltegravir C12h↓49% raltegavir Cmax ↓10%
(chelation with metal cations)
Antacids containing aluminum or magnesium reduce the concentration of raltegravir in the blood plasma. The simultaneous use of Isentress® and antacids containing aluminum or magnesium is not recommended.
Antacids containing calcium carbonate (raltegravir 400 mg 2 times a day)
raltegravir AUC↓55%
raltegravir C12h↓32% raltegavir Cmax ↓52% (chelation with metal cations)
No dose adjustment of Isentress® is required.
Histamine H2 receptor blockers and proton pump inhibitors
omeprazole (raltegravir 400 mg 2 times/day)
raltegravir AUC↑ 37%
raltegravir C12h↑24%
raltegravir Cmax↑51%
(increased solubility)
No dose adjustment of Isentress® is required.
famotidine (raltegravir 400 mg 2 times a day)
raltegravir AUC↑ 44%
raltegravir C12h↑6%
raltegravir Cmax↑60%
(increased solubility)
No dose adjustment of Isentress® is required.
Hormonal contraceptives
ethinyl estradiol norelgestromin (raltegravir 400 mg 2 times a day)
ethinyl estradiol AUC↓2%
ethinylestradiol Cmax↑6%
norelgestromin AUC↑ 14%
norelgestromin Cmax↑29%
Dose adjustment of Isentress® or hormonal contraceptives (estrogen- and/or progestogen-containing) is not required.
Opioid analgesics
methadone (raltegravir 400 mg 2 times a day)
methadone AUC ↔
methadone Cmax ↔
No dosage adjustment of Isentress® or methadone is required.
Overdose
Overdose
No specific symptoms of an overdose of Isentress® have been identified. Raltegravir was well tolerated by healthy volunteers at 1600 mg once a day and 800 mg twice a day, without any toxicity. A single dose of 1800 mg/day did not produce toxic effects in phase II/III studies. Based on the available data, it can be concluded that raltegravir is well tolerated in doses up to 800 mg 2 times a day, as well as when taken with drugs that increase its exposure by 50-70% (for example, tenofovir and atazanavir). Raltegravir has a wide therapeutic index, so the potential for toxicity due to overdose is limited.
Treatment: in case of overdose, it is necessary to follow standard recommendations, such as removing unabsorbed drug from the gastrointestinal tract, monitoring vital signs, including ECG, and prescribing symptomatic therapy. There is no data on the effectiveness of dialysis in case of overdose with Isentress®.
Storage conditions
Storage conditions
The drug should be stored out of the reach of children, in a dry place at a temperature not exceeding 30°C in tightly closed original packaging with a desiccant agent.
Shelf life
Shelf life
year. Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
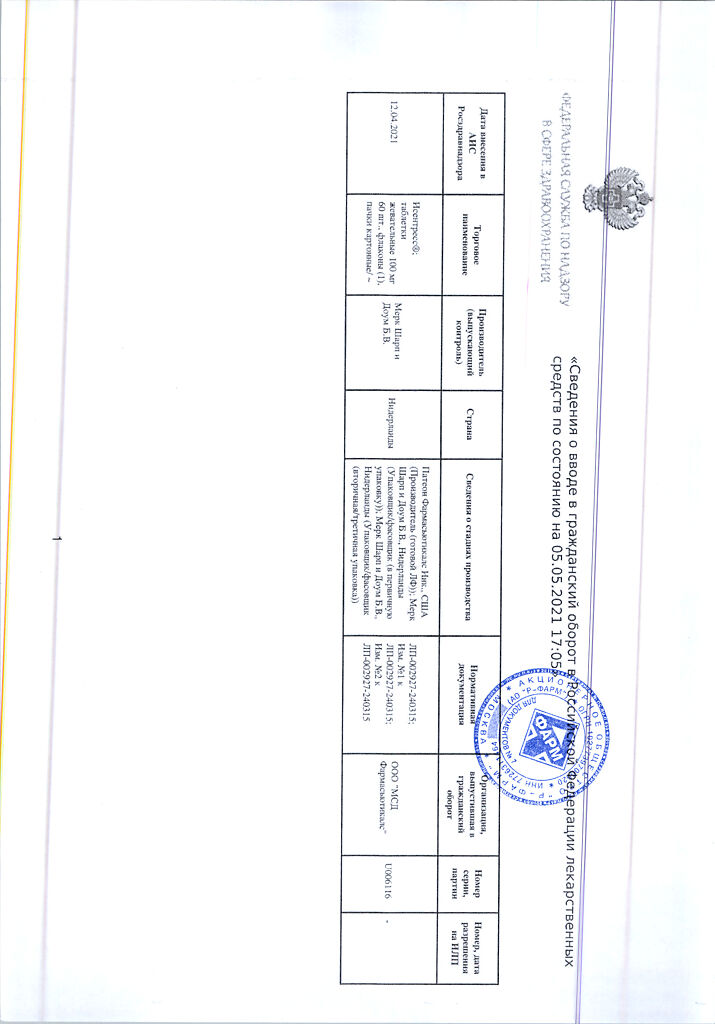
Patheon Pharmaceuticals Inc., USA
Additional information
| Shelf life | year. Do not use after the expiration date printed on the package. |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children, dry place at a temperature not exceeding 30 ° C in a tightly closed original package with a desiccant. |
| Manufacturer | Pateon Pharmaceuticals Inc, USA |
| Medication form | chewable tablets |
| Brand | Pateon Pharmaceuticals Inc |
Related products
Buy Isentress, 100 mg 60 pcs. with delivery to USA, UK, Europe and over 120 other countries.