No products in the cart.
Description
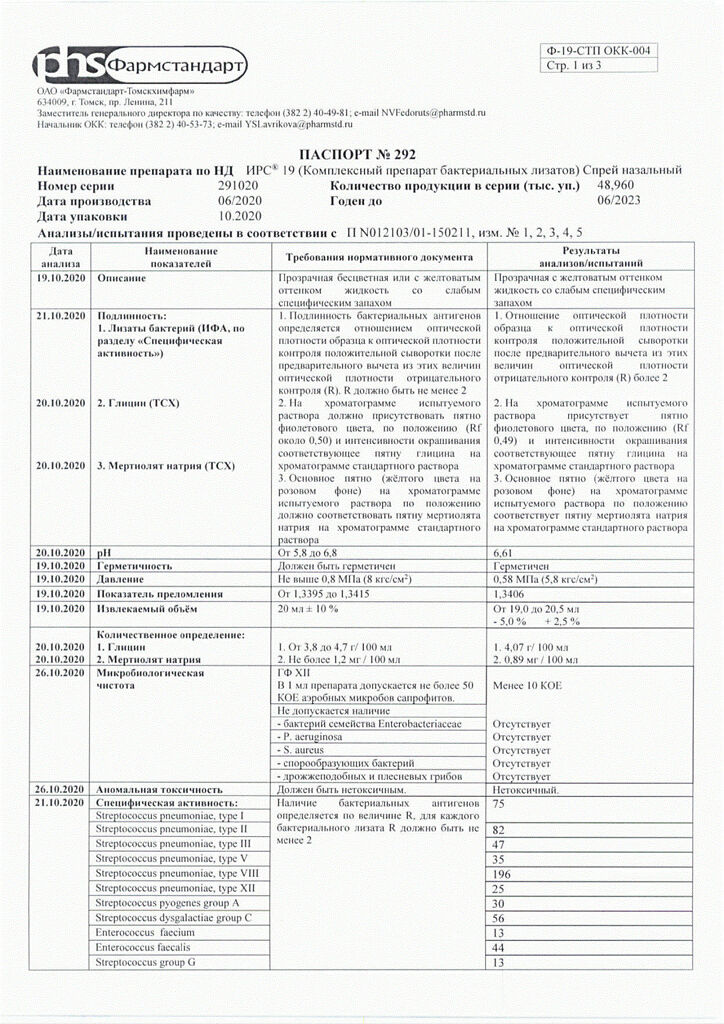
Enhancing specific and nonspecific immunity.
When sprayed, a fine aerosol is formed, which covers the nasal mucosa, resulting in rapid development of a local immune response. Specific protection is due to locally formed antibodies of the class of secretory immunoglobulins type A (IgA) which prevent the fixation and reproduction of infectious agents on the mucosa.
Nonspecific immunoprotection is manifested by increased phagocytic activity of macrophages, increased content of lysozyme.
Indications
Indications
treatment of acute, treatment and prevention of chronic diseases of the upper respiratory tract and bronchi;
restoration of local immunity after viral infections;
preparation for planned surgical intervention on ENT organs and in the postoperative period.
IRS®19 can be prescribed to both adults and children from 3 months of age.
Pharmacological effect
Pharmacological effect
Increases specific and nonspecific immunity.
When sprayed, a fine aerosol is formed that covers the nasal mucosa, which leads to the rapid development of a local immune response. Specific protection is due to locally formed antibodies of the class of secretory immunoglobulins type A (IgA), which prevent the fixation and proliferation of infectious agents on the mucosa.
Nonspecific immunoprotection manifests itself in an increase in the phagocytic activity of macrophages and an increase in the content of lysozyme.
Special instructions
Special instructions
At the beginning of treatment, reactions such as sneezing and increased nasal discharge may occur. As a rule, they are short-term in nature. If these reactions become severe, the frequency of administration of the drug should be reduced or discontinued.
At the beginning of treatment, in rare cases, an increase in body temperature ≥39°C is possible. In this case, the drug should be discontinued. However, this condition should be distinguished from an increase in body temperature, accompanied by malaise, which may be associated with the development of diseases of the ENT organs.
If clinical symptoms of a bacterial infection appear, systemic antibiotics should be considered.
When prescribing the drug IRS to 19 patients with bronchial asthma, an increase in attacks is possible. In this case, it is recommended to stop treatment and not take drugs of this class in the future.
Impact on the ability to drive vehicles and operate machinery
IRS 19 does not affect psychomotor functions associated with driving vehicles or operating machines and mechanisms.
Active ingredient
Active ingredient
Bacterial lysates
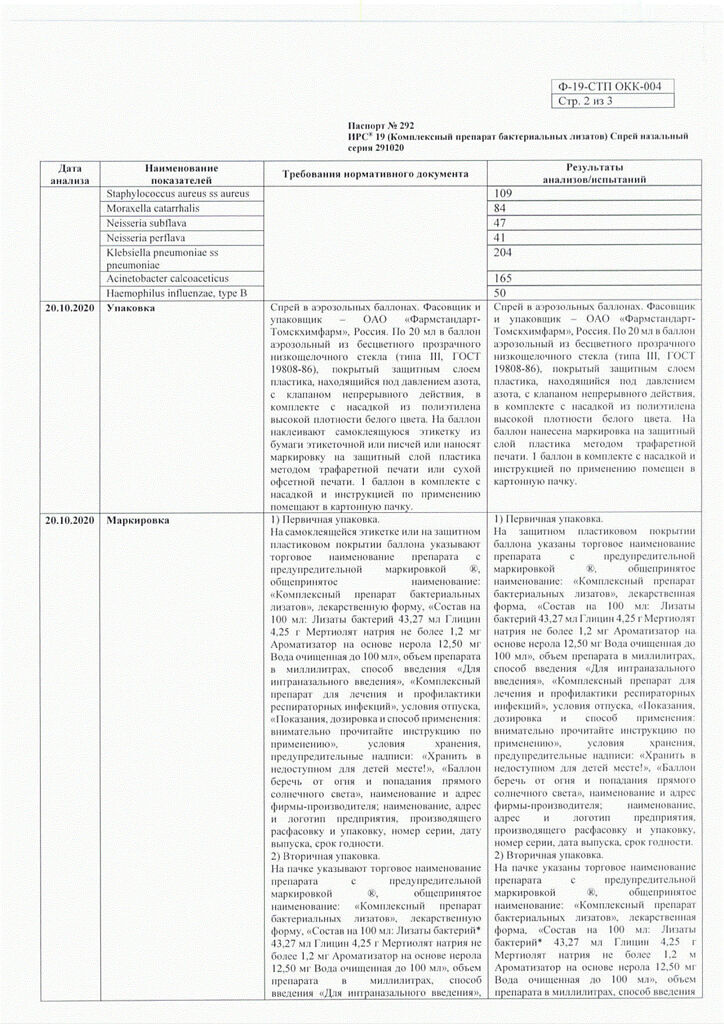
Composition
Composition
Active substances:
bacterial lysates 43.27 ml,
incl. Streptococcus pneumoniae type I1.11 ml
Streptococcus pneumoniae type II1.11 ml
Streptococcus pneumoniae type III1.11 ml
Streptococcus pneumoniae type V1.11 ml
Streptococcus pneumoniae type VIII1.11 ml
Streptococcus pneumoniae type XII1.11 ml
Haemophilus influenzae type B3.33 ml
Klebsiella pneumoniae ss pneumoniae6.66 ml
Staphylococcus aureus ss aureus9.99 ml
Acinetobacter calcoaceticus3.33 ml
Moraxella catarrhalis2.22 ml
Neisseria subflava2.22 ml
Neisseria perflava2.22 ml
Streptococcus pyogenes group A1.66 ml
Streptococcus dysgalactiae group C1.66 ml
Enterococcus faecium0.83 ml
Enterococcus faecalis0.83 ml
Streptococcus group G1.66 mg
Excipients:
glycine – 4.25 g,
sodium merthiolate – no more than 1.2 mg,
nerol-based flavoring (linalol, alpha-terpineol, geraniol, methyl anthranilate, limonene, geranyl acetate, linalyl acetate, diethylene glycol monoethyl ether, phenylethyl alcohol) – 12.5 mg,
purified water – up to 100 ml.
Pregnancy
Pregnancy
Not recommended during pregnancy (no data on the potential for teratogenic or toxic effects on the fetus).
Contraindications
Contraindications
autoimmune diseases;
hypersensitivity to the components of the drug.
Side Effects
Side Effects
Dermatological reactions: rarely – erythema-like and eczema-like reactions; in isolated cases – thrombocytopenic purpura and erythema nodosum.
Allergic reactions: rarely – urticaria, angioedema.
From the respiratory system: rarely – asthma attacks and cough, at the beginning of treatment – nasopharyngitis, sinusitis, laryngitis, bronchitis.
From the digestive system: rarely (at the beginning of treatment) – nausea, vomiting, abdominal pain, diarrhea.
Other: rarely (at the beginning of treatment) – increased body temperature (>39°C) for no apparent reason.
Side effects may or may not be related to the action of the drug. If the above symptoms appear, it is recommended to consult a doctor.
Interaction
Interaction
Cases of negative interaction of the drug IRS 19 with other drugs are unknown.
If clinical symptoms of a bacterial infection appear, antibiotics may be prescribed while the use of IRS 19 continues.
Overdose
Overdose
There are no known cases of overdose
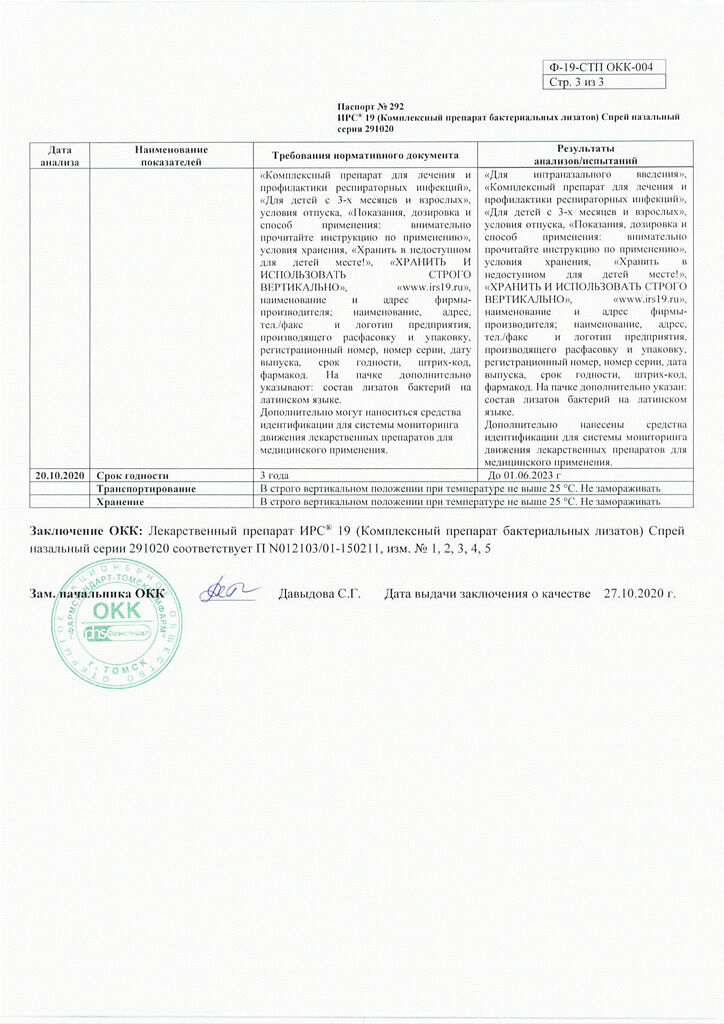
Storage conditions
Storage conditions
The drug should be stored out of the reach of children, in a strictly vertical position at a temperature not exceeding 25°C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Mylan Laboratories SAS, France
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children, in a strictly vertical position at a temperature not exceeding 25°C |
| Manufacturer | Mylan Laboratories SAS, France |
| Medication form | nasal spray |
| Brand | Mylan Laboratories SAS |
Related products
Buy IRS-19, spray 20 ml with delivery to USA, UK, Europe and over 120 other countries.