No products in the cart.
Irifrin, eye drops 2.5% 5 ml
€18.49 €15.41
Description
It has a pronounced stimulatory effect on postsynaptic alpha-adrenoreceptors, has little effect on the beta-adrenoreceptors of the heart. It has a vasoconstrictor effect (vasopressor effect of phenylephrine is weaker than that of norepinephrine, but longer), has almost no chrono- and inotropic action.
After instillation phenylephrine contracts the dilator of the pupil and smooth muscles of conjunctival arterioles, thus causing pupil dilation and constriction of conjunctival vessels, improves outflow of intraocular fluid (as phenylephrine has little effect on the ciliary muscle, mydriasis occurs without cycloplegia).Phenylephrine easily penetrates into the tissues of the eye, pupil dilation occurs within 10-60 minutes after a single injection and persists for 4-6 hours.
Due to significant contraction of the dilator of the pupil 30-45 minutes after phenylephrine instillation, pigment particles from the iris pigment sheet may be detected in the moisture of the anterior chamber of the eye.
Phenylephrine suspended in the chamber humor must be differentiated from manifestations of anterior uveitis or from blood cell infiltration into the anterior chamber humor.When used topically in normal doses, phenylephrine has no significant stimulating effect on the CNS.
Indications
Indications
Iridocyclitis (to prevent the occurrence of posterior synechiae and reduce exudation from the iris);
for pupil dilation during ophthalmoscopy and other diagnostic procedures necessary to monitor the condition of the posterior segment of the eye;
to conduct a provocative test in patients with a narrow anterior chamber angle profile and suspected angle-closure glaucoma;
Pharmacological effect
Pharmacological effect
It has a pronounced stimulating effect on postsynaptic alpha-adrenergic receptors, and has a weak effect on beta-adrenergic receptors of the heart. It has a vasoconstrictor effect (the vasopressor effect of phenylephrine is weaker than that of norepinephrine, but longer), and has virtually no chrono- and inotropic effects.
After instillation, phenylephrine contracts the pupillary dilator and smooth muscles of the arterioles of the conjunctiva, thereby causing pupil dilation and constriction of the conjunctival vessels, improves the outflow of intraocular fluid (since phenylephrine has a slight effect on the ciliary muscle, mydriasis occurs without cycloplegia). Phenylephrine easily penetrates the eye tissue, pupil dilation occurs. within 10-60 minutes after a single instillation and persists for 4-6 hours.
Due to a significant contraction of the pupil dilator, 30-45 minutes after instillation of phenylephrine, pigment particles from the pigment leaf of the iris may be detected in the aqueous humor of the anterior chamber of the eye.
Suspension in the chamber humor must be differentiated from manifestations of anterior uveitis or from the entry of blood cells into the humor of the anterior chamber. When applied topically in usual doses, phenylephrine does not have a significant stimulating effect on the central nervous system.
Special instructions
Special instructions
Irifrin should be used with caution in patients with diabetes mellitus due to the risk of developing increased blood pressure associated with impaired autonomic regulation, as well as in elderly patients due to an increased risk of reactive miosis.
Active ingredient
Active ingredient
Phenylephrine
Composition
Composition
Eye drops
Active ingredient:
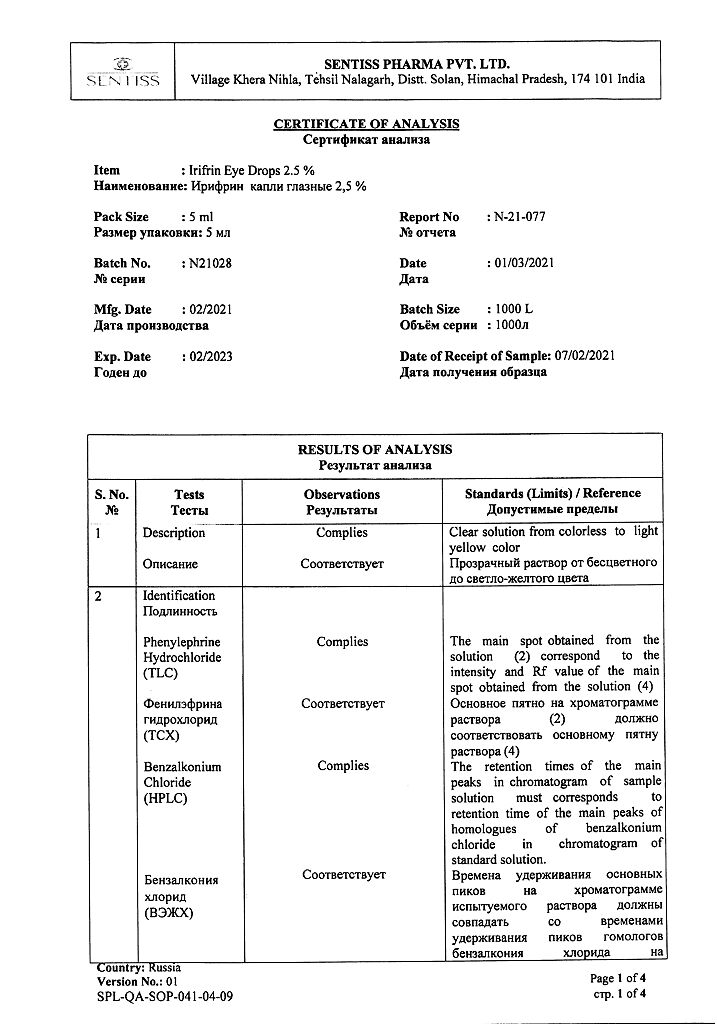
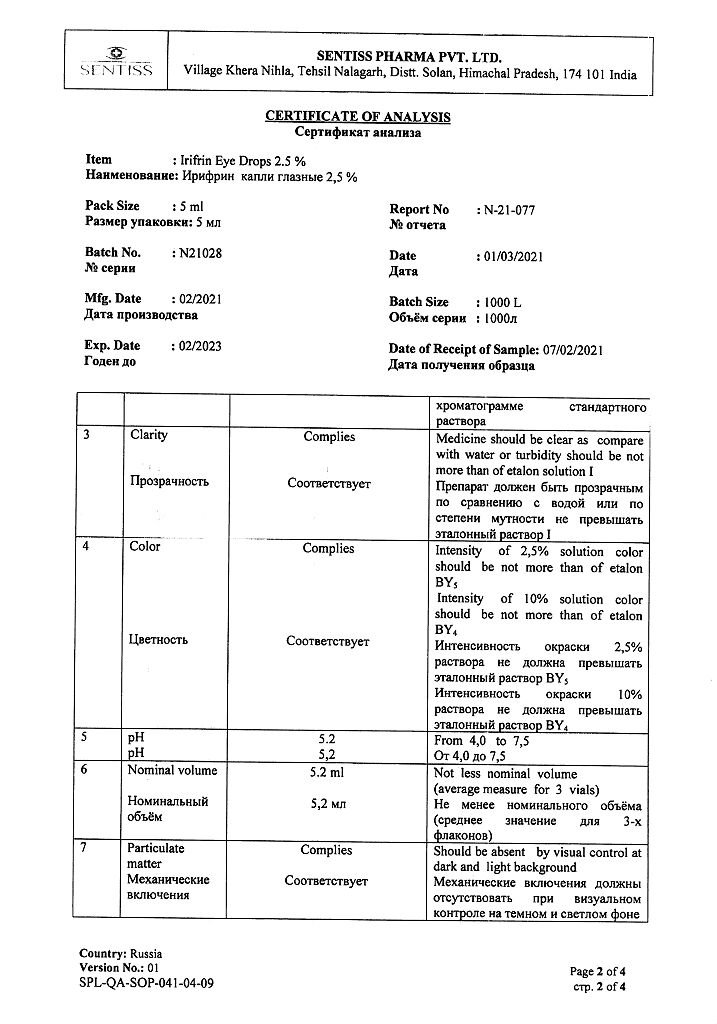
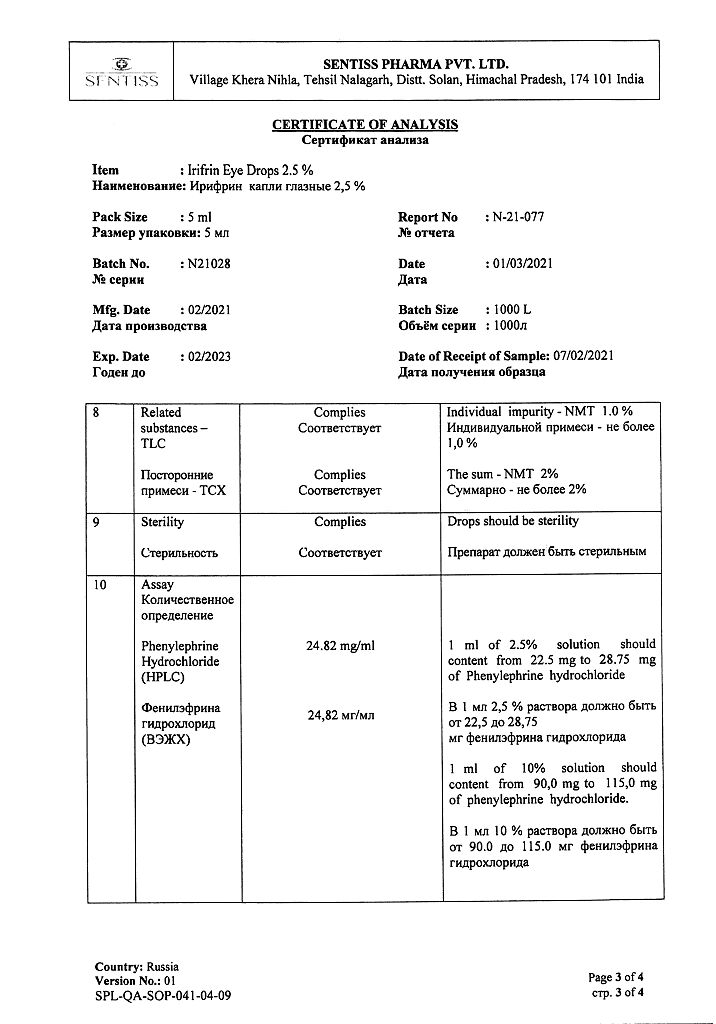
Phenylephrine hydrochloride 25 mg.
Excipients:
Benzalkonium chloride,
disodium edetate,
sodium hydroxide,
sodium metabisulfide,
citric acid,
sodium citrate dihydrate,
water d/i.
Contraindications
Contraindications
Hypersensitivity, narrow-angle or closed-angle glaucoma, old age, severe disorders of the cardiovascular system or cerebrovascular system; for additional dilation of the pupil during surgical operations in patients with a violation of the integrity of the eyeball, as well as in cases of impaired tear production; hyperthyroidism, hepatic porphyria, congenital deficiency of glucose-6-phosphate dehydrogenase; 10% solution – children’s age (up to 12 years), arterial aneurysm; 2.5% solution – for children with reduced body weight.
With caution.
Pregnancy, lactation, simultaneous use with MAO inhibitors (including within 21 days after stopping their use).
Side Effects
Side Effects
From the organ of vision: conjunctivitis, periorbital edema; Possible burning sensation at the beginning of use, blurred vision, irritation, discomfort, lacrimation, increased intraocular pressure.
The day after using Irifrin®, reactive miosis is possible. With repeated instillations of the drug during this period, mydriasis may be less pronounced than the day before. This effect is more common in elderly patients.
Due to the significant contraction of the pupil dilator under the influence of phenylephrine, 30-45 minutes after instillation, pigment particles from the pigment leaf of the iris can be detected in the moisture of the anterior chamber of the eye. Suspension in the chamber fluid must be differentiated from the appearance of anterior uveitis or from the ingress of blood cells into the fluid of the anterior chamber.
From the cardiovascular system: possible palpitations, tachycardia, arrhythmia (including ventricular), arterial hypertension, reflex bradycardia, occlusion of the coronary arteries, pulmonary embolism.
Dermatological reactions: contact dermatitis.
Rarely, when using Irifrin in the form of eye drops 10%, the development of serious disorders of the cardiovascular system, including myocardial infarction, vascular collapse and intracranial hemorrhage, is observed.
Interaction
Interaction
The mydriatic effect of phenylephrine is enhanced when used in combination with atropine. Due to increased vasopressor action, tachycardia may develop.
When using Irifrin simultaneously with MAO inhibitors or within 21 days after stopping their use, there is a risk of developing an uncontrolled rise in blood pressure.
The vasopressor effect of adrenomimetic drugs can also be potentiated when used together with tricyclic antidepressants, propranolol, reserpine, guanethidine, methyldopa and m-anticholinergics.
Irifrin can potentiate the inhibitory effect on the activity of the cardiovascular system during inhalation anesthesia.
Use together with sympathomimetics may enhance the cardiovascular effects of phenylephrine.
Overdose
Overdose
Symptoms: systemic effects of phenylephrine may occur.
Treatment: administration of alpha-blocking agents, for example 5 to 10 mg of phentolamine IV, the injection can be repeated if necessary.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 25 °C.
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Sentiss Pharma Pvt.Ltd, India
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a light-protected place at a temperature not exceeding 25 °C. |
| Manufacturer | Sentiss Pharma Pvt.Ltd, India |
| Medication form | eye drops |
| Brand | Sentiss Pharma Pvt.Ltd |
Related products
Buy Irifrin, eye drops 2.5% 5 ml with delivery to USA, UK, Europe and over 120 other countries.