No products in the cart.
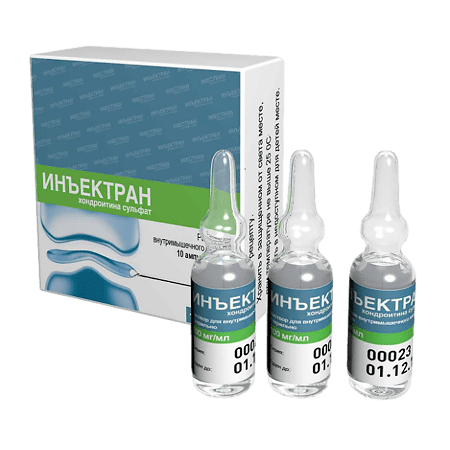
Injectran, 100 mg/ml 2 ml 10 pcs
€55.17 €45.97
Description
Chondroitin sulfate is the main component of proteoglycans, which together with the collagen fibers make up the cartilage matrix.
It has chondrostimulating, regenerating, anti-inflammatory and analgesic effect. Chondroitin sulfate is involved in the construction of the basic substance of cartilage and bone tissue.
It has chondroprotective properties, enhances metabolic processes in hyaline and fibrous cartilage and subchondral bone, inhibits the activity of enzymes that cause degradation (destruction) of articular cartilage; stimulates the production of proteoglycans by chondrocytes; influences phosphorus-calcium metabolism in cartilage tissue, stimulates its regeneration, participates in the construction of the basic substance of bone and cartilage tissue.
It has anti-inflammatory and analgesic properties, prevents release of inflammatory mediators and pain factors into synovial fluid through synoviocytes and synovial macrophages, inhibits secretion of leukotrienes and prostaglandins. The drug prevents degeneration of connective tissue and reduces calcium loss, accelerates the process of bone tissue regeneration.
Chondroitin sulfate slows the progression of osteoarthritis and osteochondrosis. Helps restore the joint capsule and cartilage surfaces of joints, prevents collapse of connective tissue and normalizes the production of joint fluid.
The clinical effect is manifested by improved joint mobility, a decrease in the intensity of pain, and the therapeutic effect is maintained for a long time after the end of the course of therapy. In the treatment of degenerative changes in the joints accompanied by secondary synovitis the effect can be observed within 2-3 weeks after the start of the course.
With structural similarity to heparin, it can potentially prevent the formation of fibrin clots in the synovial and subchondral microcirculatory bed.
Pharmacokinetics
Intake and distribution
Chondroitin sulfate sodium is easily absorbed when administered in m/m. Within 30 min after I / M administration it is found in blood in high concentrations; after 15 min – in synovial fluid. Cmax in blood plasma is reached 1 hour after injection, then drug concentration gradually decreases during 2 days.
The drug mainly accumulates in the cartilage (Cmax in articular cartilage is reached after 48 hours); the synovial membrane is not an obstacle to penetration of the drug into the joint cavity.
Elimination
Extracted mainly by the kidneys within 24 hours.
Indications
Indications
Degenerative-dystrophic diseases of the joints and spine:
– osteoarthritis of the peripheral joints;
– intervertebral osteochondrosis and osteoarthritis.
To accelerate the formation of the bone callus in fractures.
Active ingredient
Active ingredient
Composition
Composition
Associates:
sodium disulfite – 2 mg,
methyl parahydroxybenzoate – 0.5 mg,
1M sodium hydroxide solution – to pH 6.0-7.5,
d/i water – up to 1 ml.
How to take, the dosage
How to take, the dosage
I/m, 1 ml every other day. If tolerated well, the dose is increased to 2 ml starting with the fourth injection. The course of treatment is 25-35 injections. If necessary, repeated course of treatment may be carried out after 6 months. The duration of repeated courses of treatment is determined by the doctor.
For the formation of the bone callus the course of treatment is 3-4 weeks (10-14 injections a day).
Interaction
Interaction
The effect of indirect anticoagulants, antiaggregants and fibrinolytic agents may be enhanced, which requires more frequent monitoring of blood clotting parameters when used together.
It shows synergistic effect when used simultaneously with glucosamine and other chondroprotectors.
Special Instructions
Special Instructions
In case of allergic reactions or hemorrhagia, treatment should be discontinued.
Pediatric use
There are currently no data on the efficacy and safety of chondroitin sulfate in children.
Impact on ability to drive and operate vehicles
In the recommended dose range, no effect on concentration and rapid psychomotor reactions has been found.
When taking high doses, caution is recommended with regard to driving, operating machinery, and engaging in other potentially hazardous activities requiring increased concentration and quick psychomotor reactions.
Contraindications
Contraindications
– hypersensitivity to chondroitin sulfate;
– bleeding, susceptibility to bleeding;
– thrombophlebitis;
– pregnancy;
– breastfeeding (breastfeeding should be discontinued during treatment);
– childhood (data on efficacy and safety are not available).
Side effects
Side effects
When using the drug in persons with hypersensitivity to the drug, the following disorders may occur.
Immune system disorders: allergic reactions, angioedema.
Skin and subcutaneous fatty tissue disorders: skin rash, itching, erythema, urticaria, dermatitis.
Digestive system disorders: dyspeptic symptoms.
Local reactions: redness, itching, hemorrhages are possible in the injection site.
Overdose
Overdose
There are currently no reported cases of overdose of Injectran.
Symptoms: it can be assumed that if the daily dose is exceeded, the side effects of the drug may increase.
Treatment: conduct symptomatic therapy.
Similarities
Similarities
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children, protected from light, at a temperature not exceeding 25 ° C. |
| Manufacturer | Ellara, Russia |
| Medication form | solution |
| Brand | Ellara |
Related products
Buy Injectran, 100 mg/ml 2 ml 10 pcs with delivery to USA, UK, Europe and over 120 other countries.