No products in the cart.
Ilomedin, 20 µg/ml concentrate 1 ml 5 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
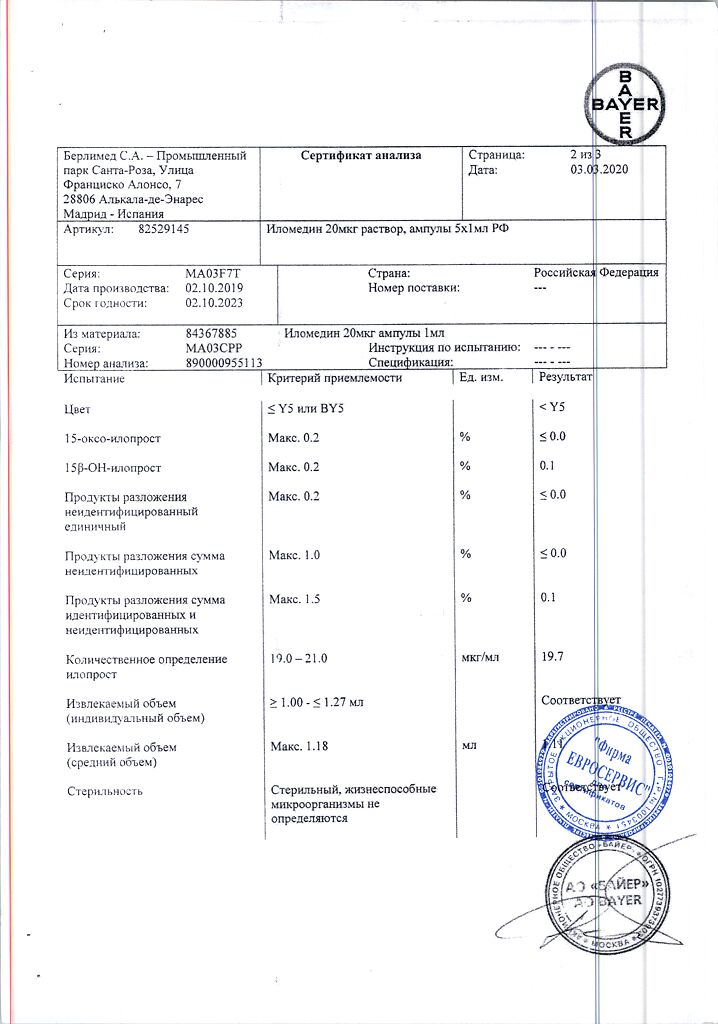
Ilomedin is an anti-aggregative.
Pharmacodynamics
Iloprost is a synthetic analog of prostacyclin, inhibits platelet aggregation, adhesion and release reaction; dilates arterioles and venules; increases capillary density (restores impaired microcirculation by inducing vasodilation, inhibition of platelet activation, endothelial repair and protection, activation of endogenous fibrinolysis and correction of imbalance in cytokine system) and reduces increased vascular permeability due to mediators such as serotonin or histamine in microcirculation system; activates endogenous fibrinolysis; shows anti-inflammatory effect Inhibits leukocyte adhesion and migration after endothelial damage, as well as leukocyte accumulation in damaged tissue; reduces production of tumor necrosis factor (TNF-alpha;).
Pharmacokinetics
Distribution. Css in blood plasma is reached very rapidly, 10-20 min after the start of v/v infusion. Its time to reach is linearly dependent on the infusion rate, at an infusion rate of 3 ng/kg/min a concentration of approximately (135±24) pg/ml is reached. After the end of infusion, plasma concentration of iloprost decreases very rapidly (this is due to its very high metabolic rate). Metabolic clearance is approximately (20±5) ml/kg/min. T1/2 from plasma in the terminal phase of distribution is about 0.5 h. In 2 h after cessation of infusion, the drug content is less than 10% of Css. The binding to plasma albumin is 60%.
Metabolism and elimination. Iloprost is metabolized mainly by B-oxidation of the side carboxyl chain. The substance is not excreted from the body unchanged. The main metabolite, tetranoriloprost, is found in the urine in free form and in 4 conjugated forms of diastereoisomers. As experiments on animals have shown, tetranoriloprost is pharmacologically inactive. Results of in vitro studies indicate a similar nature of iloprost metabolism in the lungs after IV injection or inhalation.
Evacuation. Excretion of iloprost after IV infusion in subjects with normal renal and hepatic function is in most cases characterized by a biphasic profile with an average T1/2 duration of 3-5 and 15-30 minutes, respectively. Total clearance of iloprost is approximately 20 ml/kg/min, indicating that the metabolism of iloprost occurs partially outside the liver.
A mass balance study was performed using 3H-labeled iloprost in healthy subjects. After IV infusion, excretion of total radioactivity was 81%, with 68% excreted in the urine and 12% in the feces. The elimination of metabolites from plasma and their excretion with the urine are biphasic, with a T1/2 from plasma being about 2 h in the first phase and about 5 h in the second, and 2 and 18 h for urine, respectively.
Renal insufficiency. In a study using IV infusions of iloprost, it has been shown that patients with end-stage renal failure who receive intermittent dialysis treatment have significantly lower clearance (mean clearance = (5±2) ml/min/kg) than patients with renal failure who do not receive dialysis treatment (mean clearance = (18±2) ml/min/kg).
Liver dysfunction. Because iloprost is extensively metabolized in the liver, changes in hepatic function affect plasma concentrations of the drug. The results of a study with intravenous administration of the drug included data from 8 patients with cirrhosis. Mean clearance of iloprost was calculated to be 10 ml/min/kg.
Age and sex. The pharmacokinetics of iloprost are independent of the patient’s age and sex.
Indications
Indications
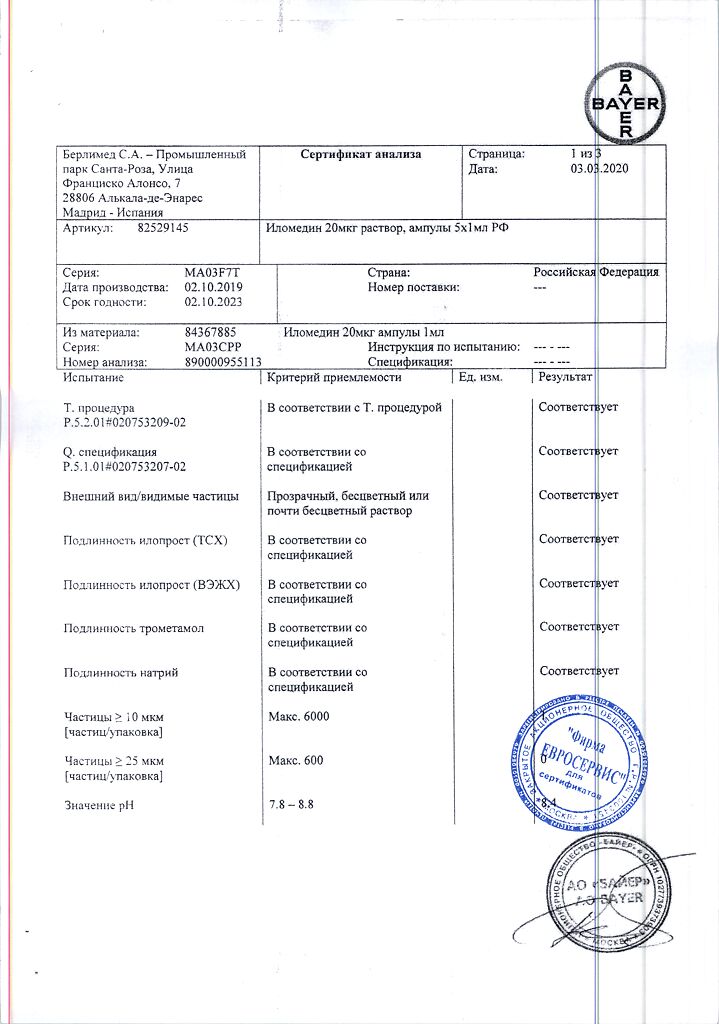
thromboangiitis obliterans (Buerger’s disease) in late stages with critical limb ischemia in cases where there are no indications for revascularization;
severe forms of peripheral arterial occlusive disease, especially in cases of risk of amputation and when vascular surgery or angioplasty is not possible;
severe Raynaud’s syndrome, leading to disability, not amenable to treatment with other drugs.
Pharmacological effect
Pharmacological effect
Ilomedin is an antiaggregant.
Pharmacodynamics
Iloprost is a synthetic analogue of prostacyclin, inhibits platelet aggregation, adhesion and platelet release reaction; dilates arterioles and venules; increases capillary density (restores impaired microcirculation by inducing vasodilation, inhibiting platelet activation, restoring and protecting the endothelium, activating endogenous fibrinolysis and correcting imbalances in the cytokine system) and reduces increased vascular permeability caused by mediators such as serotonin or histamine in the microcirculation system; activates endogenous fibrinolysis; exhibits an anti-inflammatory effect: suppresses the adhesion and migration of leukocytes after endothelial damage, as well as the accumulation of leukocytes in damaged tissue, reduces the production of tumor necrosis factor (TNF-alpha;).
Pharmacokinetics
Distribution. Css in blood plasma is achieved very quickly, 10–20 minutes after the start of intravenous infusion. The time to achieve it depends linearly on the infusion rate; at an infusion rate of 3 ng/kg/min, a concentration approximately equal to (135 ± 24) pg/ml is achieved. After the end of the infusion, the concentration of iloprost in plasma decreases very quickly (this is due to the very high intensity of its metabolism). Metabolic clearance is approximately (20±5) ml/kg/min. T1/2 from blood plasma in the terminal distribution phase is about 0.5 hours. 2 hours after stopping the infusion, the drug content is less than 10% of Css. The connection with blood plasma albumin is 60%.
Metabolism and elimination. Iloprost is metabolized mainly by B-oxidation of the carboxyl side chain. The substance is not excreted from the body unchanged. The main metabolite, tetranoryloprost, is found in urine in free form and in 4 conjugated forms of diastereoisomers. Experiments on animals have shown that tetranoryloprost is pharmacologically inactive. The results of in vitro studies indicate a similar pattern of metabolism of iloprost in the lungs after intravenous administration or inhalation.
Excretion. Elimination of iloprost after intravenous infusion in subjects with normal renal and hepatic function in most cases is characterized by a biphasic profile with average T1/2 durations of 3-5 and 15-30 minutes, respectively. The total clearance of iloprost is approximately 20 ml/kg/min, indicating that iloprost metabolism occurs partially outside the liver.
A mass balance study was conducted using 3H-labeled iloprost in healthy subjects. After IV infusion, total radioactivity excretion was 81%, with 68% excreted in urine and 12% in feces. Elimination of metabolites from plasma and their excretion in urine have a two-phase character, with T1/2 from plasma in the first phase being about 2 hours, in the second – about 5 hours, and for urine – 2 and 18 hours, respectively.
Kidney failure. In a study using IV infusions of iloprost, it was shown that patients with end-stage renal disease receiving intermittent dialysis treatment had significantly lower clearance (mean clearance = (5 ± 2) ml/min/kg) than patients with renal failure not receiving dialysis treatment (mean clearance = (18 ± 2) ml/min/kg).
Liver dysfunction. Since iloprost is extensively metabolized in the liver, changes in hepatic function affect plasma concentrations of the drug. The results of the study with intravenous administration of the drug included data from 8 patients suffering from liver cirrhosis. The average clearance of iloprost was calculated to be 10 ml/min/kg.
Age and no. The pharmacokinetics of iloprost does not depend on the age and gender of the patient.
Active ingredient
Active ingredient
Iloprost
Composition
Composition
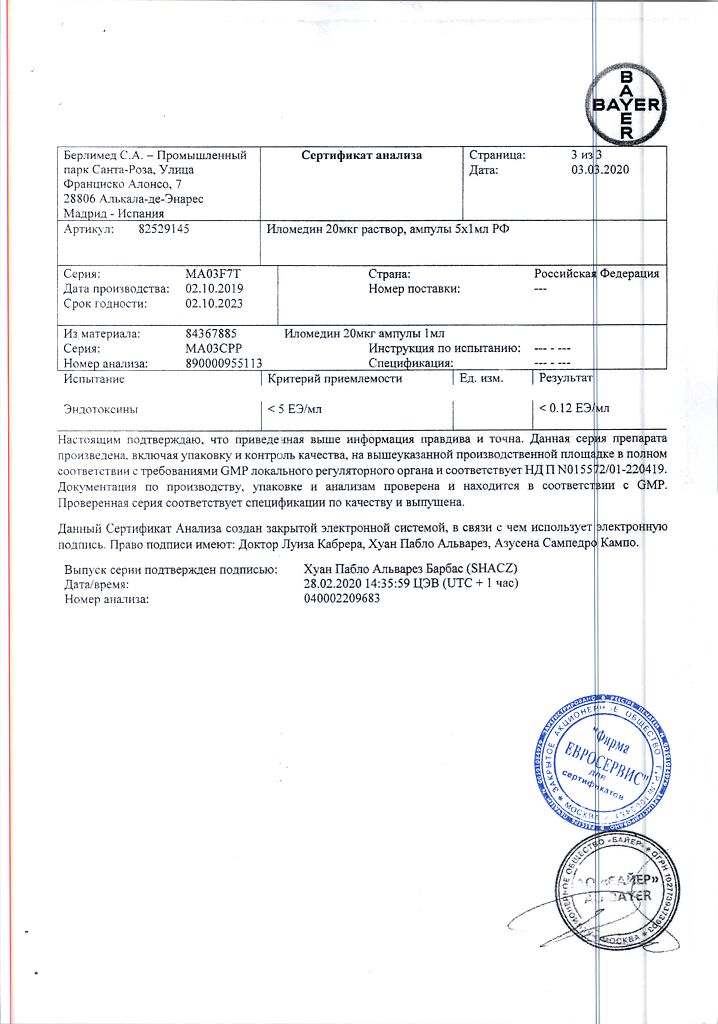
Active ingredient:
iloprost trometamol 0.027 mg (corresponding to 0.02 mg (20 mcg) iloprost);
Excipients:
trometamol – 0.235 mg;
ethanol 96% – 1.62 mg;
sodium chloride – 9 mg;
hydrochloric acid solution 1M – 0.76 mg;
water for injection – 990.758 mg
Pregnancy
Pregnancy
Ilomedin should not be prescribed to women during pregnancy and lactation. There are no data on the use of iloprost in pregnant women. Preclinical studies suggest that iloprost causes fetal toxicity in rats but not in rabbits or monkeys. Due to the fact that the potential risk of therapeutic use of iloprost during pregnancy is unknown, women of fertile age should use reliable contraception when treating iloprost.
There is currently no evidence that iloprost passes into breast milk in humans, but since there is evidence that iloprost can pass into milk in small amounts in rats, it should not be administered to nursing mothers.
Contraindications
Contraindications
hypersensitivity to iloprost or other components of the drug;
pathological conditions in which the effect of iloprost on platelets may increase the risk of bleeding (for example, acute gastric or duodenal ulcer, trauma, intracranial bleeding);
severe coronary heart disease or unstable angina; myocardial infarction within the last 6 months; acute heart failure or chronic congestive heart failure stage II–IV (according to the New York Heart Association classification); severe heart rhythm disturbances;
suspicion of congestion in the pulmonary circulation;
pregnancy;
lactation period.
With caution: patients with cerebrovascular accident in the last 3 months (for example, transient ischemic disorder, stroke). Such patients require a careful assessment of the benefit-risk ratio of treatment (see also Contraindications – risk of bleeding, such as intracranial bleeding). In case of renal failure requiring dialysis and liver cirrhosis, the excretion of iloprost is reduced (see “Dosage and Administration”). It is necessary to take measures against a further decrease in blood pressure (before starting Ilomedin therapy) in patients with initially low blood pressure values; Patients with severe heart disease should be closely monitored. The possibility of developing orthostatic hypotension should be taken into account when patients move from a horizontal to a vertical position after completing the administration of Ilomedin.
Side Effects
Side Effects
From the nervous system and sensory organs: often (more than 1%, but less than 10%) – dizziness, headache, paresthesia, hyperesthesia, tinnitus, anxiety, agitation, lethargy, apathy, drowsiness; less often (more than 0.1%, but less than 1%) – tremor, cerebrovascular disorders, depression, hallucinations, migraine, fainting, prolonged loss of consciousness, blurred vision, irritation and pain in the eyes; rarely (more than 0.01%, but less than 0.1%) – vestibular disorders; frequency unknown – confusion.
From the cardiovascular system: often (more than 1%, but less than 10%) – decreased blood pressure, bradycardia, flushing of the skin and a feeling of heat; less often (more than 0.1%, but less than 1%) – arrhythmia (including extrasystole), myocardial ischemia, myocardial infarction, deep vein thrombosis, pulmonary embolism; frequency unknown – increased blood pressure, tachycardia; in isolated cases (in elderly patients with severe atherosclerosis) – pulmonary edema.
From the respiratory system: less often (more than 0.1%, but less than 1%) – bronchial asthma; rarely (more than 0.01%, but less than 0.1%) – cough; in isolated cases (in elderly patients with severe atherosclerosis) – heart failure.
From the digestive system: more often (more than 10%) – nausea, vomiting; often (more than 1%, but less than 10%) – anorexia, diarrhea, abdominal discomfort, abdominal pain; less often (more than 0.1%, but less than 1%) – dry mouth, change in taste, tenesmus, constipation, belching, dysphagia, diarrhea, melena, rectal bleeding, jaundice.
From the musculoskeletal system: often (more than 1%, but less than 10%) – pain in the masticatory muscles, trismus, myalgia, arthralgia, muscle weakness; less often (more than 0.1%, but less than 1%) – tetany, convulsive muscle twitching, hypertonicity.
From the urinary system: lower back pain, renal colic, changes in the cellular composition of urine, dysuria.
Local reactions: often (more than 1%, but less than 10%) – skin hyperemia, pain, phlebitis at the injection site.
Other: more often (more than 10%) – sweating; often (more than 1%, but less than 10%) – local pain, generalized pain, hyperthermia, itching, fatigue, thirst; frequency unknown – allergic reactions.
Interaction
Interaction
Due to possible interactions, Ilomedin should not be mixed in the same solution with other medications.
Iloprost enhances the antihypertensive effect of β-blockers, CCBs and all vasodilators, as well as ACE inhibitors. If significant arterial hypotension occurs, blood pressure can be adjusted by reducing the dose of iloprost.
Since iloprost suppresses platelet function, its use in combination with heparin or indirect anticoagulants (coumarin derivatives) or other platelet aggregation inhibitors (acetylsalicylic acid, NSAIDs, PDE inhibitors and nitrate vasodilators, such as molsidomine) may increase the risk of bleeding. In such a case, Ilomsdin infusion should be stopped.
The use of acetylsalicylic acid at a dose of up to 300 mg/day for a course of 8 days, preceding the use of Ilomedin, did not have any effect on the pharmacokinetics of iloprost. In an animal study, it was found that iloprost may cause a decrease in tissue plasminogen activator (tPA) Css in plasma. Results from human studies indicate that iloprost infusions do not affect the pharmacokinetics of multiple oral doses of digoxin in patients, and when administered concomitantly with tPA drugs, iloprost does not affect its pharmacokinetics.
In animal experiments, the vasodilatory effect of iloprost was weakened if experimental animals had previously received GCS, but the inhibitory effect on platelet aggregation did not change. The significance of these data for the clinic has not yet been established.
Although no clinical studies have been conducted, in vitro studies examining the inhibitory potential of iloprost on the activity of cytochrome P450 enzymes have shown that significant inhibition of drug metabolism by these enzymes due to exposure to iloprost is unlikely.
Overdose
Overdose
Symptoms: Possible decrease in blood pressure, as well as headache, blood flow to the face, nausea, vomiting and diarrhea. Increased blood pressure, bradycardia or tachycardia, pain in the legs or back are possible.
Treatment: interruption of infusion, further monitoring of patients and symptomatic therapy. Specific antidotes are unknown.
Storage conditions
Storage conditions
At a temperature not exceeding 30 °C.
Shelf life
Shelf life
4 years
Manufacturer
Manufacturer
Berlimed S.A., Spain
Additional information
| Shelf life | 4 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 30 °C. |
| Manufacturer | Berlimed S.A., Spain |
| Medication form | concentrate for preparation of infusion solution |
| Brand | Berlimed S.A. |
Related products
Buy Ilomedin, 20 µg/ml concentrate 1 ml 5 pcs with delivery to USA, UK, Europe and over 120 other countries.