No products in the cart.
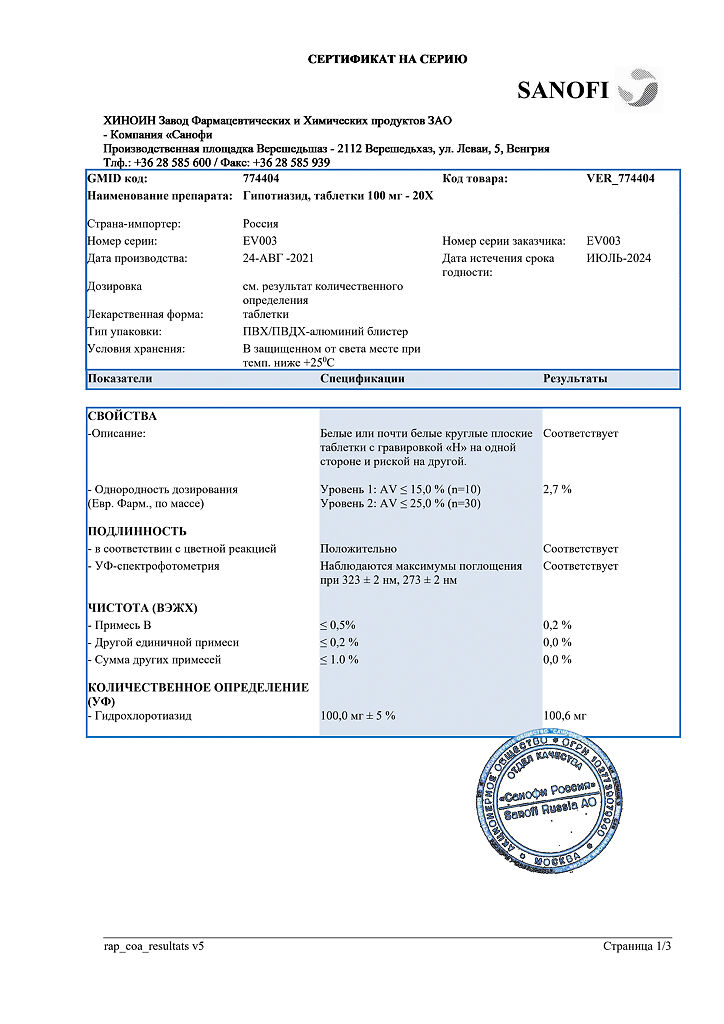
Hypothiazide, tablets 100 mg 20 pcs
€3.21 €2.67
Out of stock
(E-mail when Stock is available)
Description
Pharmgroup:
Diuretic.
Pharmic action:
Hypothiazide is a diuretic. The primary mechanism of action of thiazide diuretics is to increase diuresis by inhibiting reabsorption of sodium and chlorine ions in the initial part of the renal tubules. This leads to increased excretion of sodium and chlorine and, consequently, water. The excretion of other electrolytes, namely potassium and magnesium, also increases. At maximum therapeutic doses, the diuretic/natriuretic effect of all thiazides is approximately the same.
Natriuresis and diuresis occur within 2 h and reach maximum levels after approximately 4 h.
The thiazides also reduce carboanhydrase activity by increasing excretion of bicarbonate ions, but this effect is usually weak and has no effect on urine pH.
Hydrochlorothiazide also has hypotensive properties. Thiazide diuretics do not affect normal BP.
Pharmacokinetics:
Absorption and distribution
Hydrochlorothiazide is incompletely but fairly rapidly absorbed from the GI tract. This action persists for 6-12 hours. After oral administration in a dose of 100 mg Cmax in blood plasma is reached after 1.5-2.5 hours. At the maximum of diuretic activity (about 4 hours after intake) the plasma concentration of hydrochlorothiazide is 2 mcg/ml.
The binding to plasma proteins is 40%.
Hydrochlorothiazide penetrates the placental barrier and is excreted with breast milk.
Elimation
The primary route of excretion is by the kidneys (filtration and secretion) in unchanged form. T1/2 for patients with normal renal function is 6.4 h.
Pharmacokinetics in special clinical cases
The T1/2 for patients with moderate renal impairment is 11.5 h. T1/2 for patients with CK< 30 ml/min is 20.7 h.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
1 tablet contains:
Active ingredient:
Hydrochlorothiazide 100 mg;
Associates:
magnesium stearate;
talc;
gelatin;
corn starch;
lactose monohydrate.
How to take, the dosage
How to take, the dosage
Ingestion, after meals.
The dosage has to be adjusted individually. With constant medical supervision, the minimum effective dose is established.
With the increased loss of potassium and magnesium ions during treatment (serum potassium levels may decrease below 3.0 mmol/L), there is a need for potassium and magnesium replacement.
Adults. As an antihypertensive agent, the usual initial daily dose is 25-50 mg once, as monotherapy or in combination with other antihypertensive agents. In some patients, a starting dose of 12.5 mg, either as monotherapy or in combination, is sufficient. A minimum effective dose not exceeding 100 mg/day should be used. If Hypothiazide® is combined with other hypotensive drugs, it may be necessary to reduce the dose of the other drug in order to prevent excessive BP reduction.
The hypotensive effect is seen within 3 to 4 days, but it may take up to 3 to 4 weeks to achieve optimal effect. After the end of treatment, the hypotensive effect lasts for 1 week.
Oedema syndrome of various genesis. Usual starting dose during edema treatment is 25-100 mg of medicine once a day or once every 2 days. Depending on clinical reaction, the dose may be reduced to 25-50 mg once daily or once every 2 days. In some severe cases, doses up to 200 mg/day may be required at the beginning of treatment.
In premenstrual syndrome, the usual dose is 25 mg/day and is used from the onset of symptoms until the onset of menstruation.
In nephrogenic nonspecific diabetes, the usual daily dose of 50-150 mg (in multiple doses) is recommended.
In children. Doses should be set based on the child’s body weight. The usual pediatric daily doses are 1-2 mg/kg or 30-60 mg/m2 of body surface area, administered once daily. The total daily dose for children aged 3 to 12 years is 37.5-100 mg.
Interaction
Interaction
Combined administration of the drug with lithium salts should be avoided (renal clearance of lithium is decreased and its toxicity increases).
Cautiously use with the following drugs:
Special Instructions
Special Instructions
In long-term treatment it is necessary to carefully monitor the clinical symptoms of water-electrolyte imbalance, especially in high-risk patients: patients with cardiovascular disease and liver dysfunction; in case of severe vomiting or in case of signs of water-electrolyte imbalance such as dry mouth, thirst, weakness, lethargy, somnolence, restlessness, muscle pain or cramps, muscle weakness, hypotension, oliguria, tachycardia, complaints from the GI tract.
Hypokalemia can be avoided by using potassium-containing medications or potassium-rich foods (fruits, vegetables), especially in cases of increased potassium loss (increased diuresis, prolonged treatment) or concurrent treatment with foxglove glycosides or corticosteroid drugs.
Thiazides have been shown to increase urinary excretion of magnesium; this may lead to hypomagnesemia.
Creatinine clearance control is necessary in reduced renal function. In patients with impaired renal function, the drug may cause azotemia and cumulative effects may develop. If impaired renal function is evident, discontinuation of the drug should be considered if oliguria occurs.
Patients with impaired hepatic function or with advanced liver disease are prescribed thiazides with caution because slight changes in water-electrolyte balance as well as serum ammonium levels may cause hepatic coma.
In cases of severe cerebral and coronary sclerosis, administration of the drug requires special caution.
The treatment with thiazide drugs may impair glucose tolerance. During long-term treatment of manifest and latent diabetes mellitus, systematic monitoring of carbohydrate metabolism is necessary; it may be necessary to change the dose of hypoglycemic agents. Increased monitoring of patients with impaired uric acid metabolism is required.
Alcohol, barbiturates, narcotic analgesics increase orthostatic hypotensive effect of thiazide diuretics.
In prolonged therapy, pathological changes of parathyroid glands accompanied by hypercalcemia and hypophosphatemia have rarely been observed. Thiazides can decrease the amount of iodine bound to serum proteins without showing signs of thyroid function disorder.
In patients with lactose intolerance, gastrointestinal complaints may occur due to the presence of lactose in the tablets: Hypothiazide® 25 mg tablets contain 63 mg of lactose, Hypothiazide® 100 mg contains 39 mg of lactose.
Impact on the ability to drive and perform work requiring increased attention. In the initial stage of using the drug (the duration of this period is determined individually) it is prohibited to drive a car and perform work requiring increased attention.
Contraindications
Contraindications
– anuria;
– severe renal insufficiency (CK< 30 ml/min);
– severe hepatic insufficiency;
– difficult to control diabetes;
– Addison’s disease;
– refractory hypokalemia, hyponatremia, hypercalcemia;
– children under 3 years of age (for solid dosage form);
– hypersensitivity to components of the preparation Hypothiazide;
– hypersensitivity to sulphonamide derivatives.
The preparation should be used with caution in hypokalemia, hyponatremia, hypercalcemia, coronary artery disease, gout, lactose intolerance, usage of cardiac glycosides and in elderly patients.
Side effects
Side effects
Hypokalemia, hypomagnesemia, hypercalcemia, and hypochloremic alkalosis: dry mouth, thirst, irregular heart rhythm, mood or mental changes, cramps and muscle pain, nausea, vomiting, unusual fatigue or weakness. Hypochloremic alkalosis can cause hepatic encephalopathy or hepatic coma.
Hyponatremia: confusion, convulsions, lethargy, slowed thinking process, fatigue, agitation, muscle cramps.
Metabolic phenomena: hyperglycemia, glucosuria, hyperuricemia with the development of a gout attack. Treatment with thiazides may decrease glucose tolerance, and latent diabetes mellitus may manifest. Serum lipid levels may increase with high doses.
Gastrointestinal disorders: cholecystitis or pancreatitis, cholestatic jaundice, diarrhea, sialadenitis, constipation, anorexia.
Cardiovascular system: arrhythmias, orthostatic hypotension, vasculitis.
Nervous system and sensory system disorders: dizziness, blurred vision (temporarily), headache, paresthesias.
Hematopoietic organs: very rarely leukopenia, agranulocytosis, thrombocytopenia, hemolytic anemia, aplastic anemia.
High sensitivity reactions: urticaria, purpura, necrotizing vasculitis, Stevens-Johnson syndrome, respiratory distress syndrome (including pneumonitis and noncardiac pulmonary edema), photosensitivity, anaphylactic reactions up to and including shock.
Other events: decreased potency, impaired renal function, interstitial nephritis.
Overdose
Overdose
The most prominent manifestation of hydrochlorothiazide overdose is an acute loss of fluid and electrolytes, expressed in the following signs and symptoms:
Cardiovascular: tachycardia, decreased BP, shock.
Neuromuscular: weakness, confusion, dizziness and calf muscle spasms, paresthesia, impaired consciousness, fatigue.
Gastrointestinal: nausea, vomiting, thirst.
Renal: polyuria, oliguria or anuria (due to hemoconcentration).
Laboratory findings: hypokalemia, hyponatremia, hypochloremia, alkalosis, elevated blood urea nitrogen levels (especially in patients with renal insufficiency).
Treatment: there is no specific antidote in hydrochlorothiazide overdose.
Induction of vomiting, gastric lavage may be ways to eliminate the drug.
Absorption of the drug can be reduced by administration of activated charcoal. In cases of decreased BP or shock, reimburse CPR and electrolytes (potassium, sodium).
The water-electrolyte balance (especially serum potassium levels) and renal function should be monitored until normal values are established.
Pregnancy use
Pregnancy use
Hydrochlorothiazide penetrates the placental barrier. The use of the drug is contraindicated in the first trimester of pregnancy. In II and III trimesters of pregnancy the drug may be administered only in case of acute need when the benefit to the mother exceeds the potential risk to the fetus and/or child. There is a risk of fetal or neonatal jaundice, thrombocytopenia and other consequences.
The drug penetrates into the mother’s milk; therefore, if use of the drug is absolutely necessary, breastfeeding should be stopped.
Similarities
Similarities
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | In a light-protected place at a temperature not exceeding 25 °C. |
| Manufacturer | Hinoin Pharmaceutical and Chemical Works, Hungary |
| Medication form | pills |
| Brand | Hinoin Pharmaceutical and Chemical Works |
Related products
Buy Hypothiazide, tablets 100 mg 20 pcs with delivery to USA, UK, Europe and over 120 other countries.