No products in the cart.
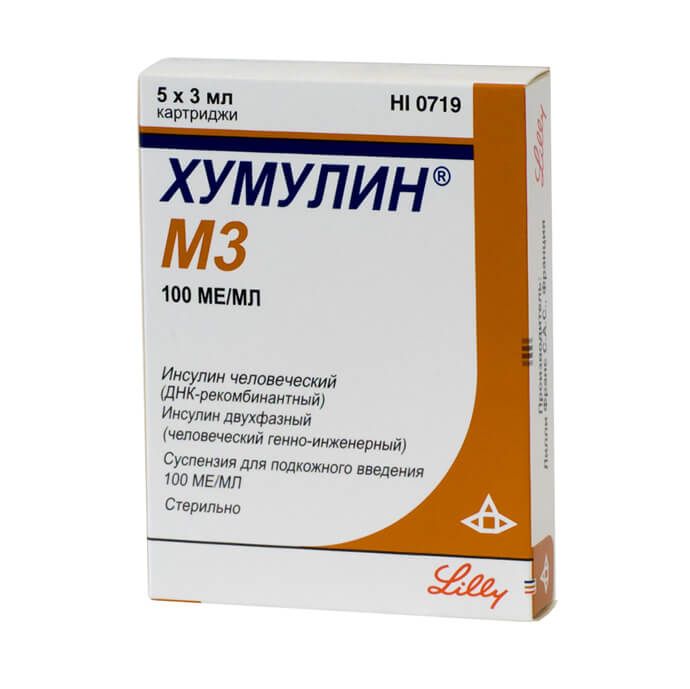
Humulin M3, 100 me/ml suspension 3 ml cartridges 5 pcs
€28.53 €24.72
Out of stock
(E-mail when Stock is available)
Description
Humulin M3 is a DNA recombinant human insulin of medium duration of action. It is a biphasic suspension (30% Humulin Regulari 70% Humulin NPH).
The main action of the drug is regulation of glucose metabolism. In addition, it has an anabolic action. In muscle and other tissues (except brain) insulin causes rapid intracellular transport of glucose and amino acids, accelerates protein anabolism. Insulin promotes conversion of glucose into glycogen in the liver, inhibits gluconeogenesis and stimulates conversion of excess glucose into fat.
Pharmacokinetics
Humulin M3 is a medium-acting insulin drug.
The onset of action of the drug is 30 minutes after administration, with maximal effect of action between 1 and 8.5 hours, and duration of action is 14-15 hours.
Individual differences in insulin activity depend on factors such as the dose, choice of injection site, and physical activity of the patient.
Indications
Indications
diabetes mellitus if there are indications for insulin therapy;
newly diagnosed diabetes mellitus;
pregnancy with type 2 diabetes mellitus (non-insulin dependent).
Pharmacological effect
Pharmacological effect
Humulin M3 is a DNA recombinant human insulin with an average duration of action. It is a two-phase suspension (30% Humulin Regulari 70% Humulin NPH).
The main effect of the drug is the regulation of glucose metabolism. In addition, it has an anabolic effect. In muscle and other tissues (with the exception of the brain), insulin causes rapid intracellular transport of glucose and amino acids and accelerates protein anabolism. Insulin promotes the conversion of glucose into glycogen in the liver, inhibits gluconeogenesis, and stimulates the conversion of excess glucose into fat.
Pharmacokinetics
Humulin M3 is an intermediate-acting insulin preparation.
The onset of action of the drug is 30 minutes after administration, the maximum effect is between 1 and 8.5 hours, the duration of action is 14-15 hours.
Individual differences in insulin activity depend on factors such as dose, choice of injection site, and physical activity of the patient.
Special instructions
Special instructions
The transition from one type of insulin to another should be carried out under the control of blood glucose levels.
In case of an overdose of insulin, if the patient is conscious, it is necessary to administer glucose orally; in case of loss of consciousness, glucose is administered intravenously or glucagon subcutaneously, intramuscularly or intravenously.
Impact on the ability to drive vehicles and operate machinery
When transferring a patient to this insulin, a temporary decrease in the speed of psychomotor reactions is possible.
When using insulin for the first time, changing its type, or in the presence of significant physical or mental stress, the speed of psychomotor reactions and the ability to concentrate may decrease.
Active ingredient
Active ingredient
Biphasic insulin (human genetically engineered)
Composition
Composition
1 ml of injection suspension contains:
active substance:
a mixture of soluble human insulin and isophane insulin suspension 100 units (soluble human insulin 30% and 70% human isophane insulin suspension)
Pregnancy
Pregnancy
During pregnancy and lactation, the need for insulin changes, which should be taken into account to maintain adequate metabolic control.
Insulin does not cross the placental barrier.
Contraindications
Contraindications
Hypoglycemia, insulinoma, increased sensitivity to this insulin.
Side Effects
Side Effects
Side effects associated with the effect on carbohydrate metabolism: hypoglycemic conditions (pallor, increased sweating, palpitations, sleep disorders, tremor).
Allergic reactions: rarely – skin rash; extremely rarely – angioedema.
Local reactions: rarely – hyperemia and itching at the injection site; with long-term use, rarely – lipodystrophy at the injection site.
Interaction
Interaction
The hypoglycemic effect of insulin is enhanced by MAO inhibitors, non-selective beta-blockers, sulfonamides, anabolic steroids, tetracyclines, clofibrate, cyclophosphamide, fenfluramine; preparations containing ethanol.
The hypoglycemic effect of insulin is reduced by oral contraceptives, glucocorticoids, thyroid hormones, thiazide diuretics, heparin, lithium preparations, tricyclic antidepressants.
Under the influence of reserpine and salicylates, it is possible to both weaken and enhance the action of insulin.
Beta-blockers, clonidine, reserpine can mask the symptoms of hypoglycemia.
Ethanol and various disinfectants can reduce the biological activity of insulin.
Overdose
Overdose
Symptoms: hypoglycemia, accompanied by lethargy, increased sweating, tachycardia, pale skin, headache, trembling, vomiting, confusion.
Under certain conditions, such as long-term diabetes or intensive control of diabetes, the warning symptoms of hypoglycemia may change.
Treatment: Mild hypoglycemia can usually be treated with oral glucose (dextrose) or sugar. Adjustments to your insulin dose, diet, or physical activity may be necessary.
Correction of moderate hypoglycemia can be carried out using intramuscular or subcutaneous administration of glucagon, followed by oral carbohydrates.
Severe conditions of hypoglycemia, accompanied by coma, convulsions or neurological disorders, are treated with intramuscular or subcutaneous administration of glucagon or intravenous administration of a concentrated solution of glucose (dextrose). After regaining consciousness, the patient must be given food rich in carbohydrates to avoid re-development of hypoglycemia.
Storage conditions
Storage conditions
Refrigerate at 2–8°C (do not freeze)
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Lilly France, France
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In the refrigerator, at 2-8 °C (do not freeze) |
| Manufacturer | Lilly France, France |
| Medication form | suspension |
| Brand | Lilly France |
Related products
Buy Humulin M3, 100 me/ml suspension 3 ml cartridges 5 pcs with delivery to USA, UK, Europe and over 120 other countries.