No products in the cart.
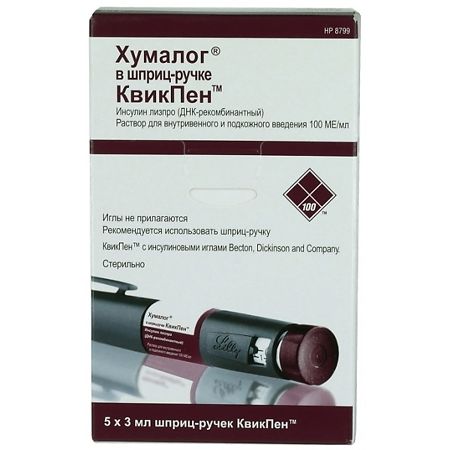
Humalog QuickPen syringe pen cartridges, 100 me/ml 3 ml QuickPen syringe pen cartridges 5 pcs
€45.14 €39.12
Description
Type 2 diabetes, Type 1 diabetes
Indications
Indications
Diabetes mellitus in adults and children, requiring insulin therapy to maintain normal blood glucose levels.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group
Hypoglycemic agent, short-acting analogue of human insulin.
ATX code
[A10AB04].
Pharmacological properties
Humalog® is a DNA recombinant analogue of human insulin. It differs from human insulin in the reverse amino acid sequence at positions 28 and 29 of the insulin B chain.
Pharmacodynamics
The main effect of insulin lispro is the regulation of glucose metabolism.
In addition, it has anabolic and anti-catabolic effects on various tissues of the body. In muscle tissue, there is an increase in the content of glycogen, fatty acids, glycerol, increased protein synthesis and an increase in amino acid consumption, but at the same time there is a decrease in glycogenolysis, gluconeogenesis, ketogenesis, and lipolysis. protein catabolism and amino acid release.
Insulin lispro has been shown to be equimolar to human insulin, but its effect is faster and lasts less. Insulin lispro has a rapid onset of action (about 15 minutes), as it has a high absorption rate, and this allows it to be administered immediately before meals (0-15 minutes before meals) in contrast to regular short-acting insulin (30-45 minutes before meals). Insulin lispro works quickly and has a shorter duration of action (2 to 5 hours) compared to regular human insulin.
In patients with type 1 and type 2 diabetes mellitus, when using insulin lispro, postprandial hyperglycemia is reduced more significantly compared to soluble human insulin.
As with all insulin products, the duration of action of insulin lispro may vary between patients or over time within the same patient and is dependent on the dose, injection site, blood supply, body temperature and physical activity.
The pharmacodynamic profile of insulin lispro in children and adolescents is similar. which is observed in adults.
The use of insulin lispro in patients with type 1 and type 2 diabetes mellitus is accompanied by a decrease in the frequency of nocturnal hypoglycemic reactions in comparison with soluble human insulin.
The glucodynamic response to insulin lispro is independent of liver or renal function.
Pharmacokinetics
After subcutaneous administration, insulin lispro is rapidly absorbed and reaches maximum concentration in the blood within 30-70 minutes.
When administered subcutaneously, the half-life of insulin lispro is approximately 1 hour.
Insulin lispro is more rapidly absorbed than soluble human insulin in patients with renal impairment. In patients with type 2 diabetes mellitus, pharmacokinetic differences are observed between insulin lispro and soluble human insulin, regardless of renal function. Insulin lispro is more rapidly absorbed and eliminated than soluble human insulin in patients with hepatic impairment.
Special instructions
Special instructions
Transferring a patient to another type or preparation of insulin should be carried out under strict medical supervision. Changes in potency, brand (manufacturer), type (Regular, NPH, etc.), species (animal, human, human insulin analogue) and/or production method (DNA recombinant insulin or animal insulin) may require dose adjustment.
In patients with hypoglycemic reactions after switching from animal insulin to human insulin, early symptoms of hypoglycemia may be less severe or different from those they experienced with their previous insulin. Uncorrected hypo- and hyperglycemic conditions can lead to loss of consciousness, coma or death.
It must be taken into account that a consequence of the pharmacodynamics of rapid-acting human insulin analogues is that if hypoglycemia develops, it may develop after injection of a rapid-acting human insulin analogue earlier than in the case of soluble human insulin.
For patients receiving short-acting and basal insulins, it is necessary to adjust the dose of both insulins to achieve optimal blood glucose concentrations throughout the day, especially at night or on an empty stomach.
Symptoms that predict hypoglycemia may change and be less pronounced with long-term diabetes mellitus, diabetic neuropathy, or treatment with drugs such as beta-blockers.
The use of inappropriate doses or discontinuation of treatment, especially in patients with type 1 diabetes mellitus, can lead to hyperglycemia and diabetic ketoacidosis, conditions that are potentially life-threatening for the patient.
Insulin requirements may be reduced in cases of renal failure, as well as in patients with liver failure as a result of decreased gluconeogenesis and insulin metabolism. However, in patients with chronic liver failure, increased insulin resistance may lead to increased insulin requirements.
The need for insulin may increase with certain diseases or emotional stress.
Insulin dosage adjustments may be required as patients increase physical activity or change their usual diet. Physical activity may increase the risk of hypoglycemia.
When using insulin drugs in combination with drugs of the thiazolidinedione group, the risk of developing edema and chronic heart failure increases, especially in patients with diseases of the cardiovascular system and the presence of risk factors for chronic heart failure.
The use of Humalog® in children instead of soluble human insulin is preferable in cases where a rapid onset of insulin action is required (for example, administering insulin immediately before meals).
To avoid possible transmission of infectious disease, each cartridge/syringe pen should only be used by one patient, even if the needle is changed.
Humalog® cartridges must be used with CE marked pens in accordance with the device manufacturer’s instructions.
Impact on the ability to drive vehicles and operate machinery
During hypoglycemia, a person’s concentration and speed of psychomotor reactions may decrease. This may pose a hazard in situations where these abilities are particularly needed (for example, driving a vehicle or operating machinery).
Patients should be advised to take precautions to prevent hypoglycemic reactions while driving vehicles and operating machinery. This is especially important for patients with mild or absent warning signs of hypoglycemia or those with frequent hypoglycemia. In such cases, the doctor must evaluate the feasibility of the patient driving vehicles and operating machinery.
Active ingredient
Active ingredient
Insulin lispro
Composition
Composition
1 ml contains:
active substance: insulin lispro 100 IU;
excipients: glycerol (glycerol) 16 mg, metacresol 3.15 mg, zinc oxide q.s. to the content of Zn++ 0.0197 mg, sodium hydrogen phosphate heptahydrate 1.88 mg, hydrochloric acid solution 10% and/or sodium hydroxide solution 10% q.s. up to pH 7.0 – 8.0. water for injection q.s. up to 1 ml.
Pregnancy
Pregnancy
Numerous data on the use of insulin lispro during pregnancy indicate the absence of an undesirable effect of the drug on pregnancy or the condition of the fetus and newborn.
During pregnancy, the main thing is to maintain good glycemic control in patients with diabetes mellitus receiving insulin treatment. Insulin requirements usually decrease during the first trimester and increase during the second and third trimesters. During and immediately after childbirth, the need for insulin may decrease dramatically.
Patients with diabetes should consult their doctor if they become or plan to become pregnant. In the case of pregnancy in patients with diabetes, the main thing is careful monitoring of glucose, as well as general health.
For patients with diabetes mellitus, adjustment of the dose of insulin, diet, or both may be necessary during breastfeeding.
Contraindications
Contraindications
Hypersensitivity to insulin lispro or any excipient;
Hypoglycemia.
Side Effects
Side Effects
Hypoglycemia is the most common adverse event when treating patients with diabetes with insulin. Severe hypoglycemia can lead to loss of consciousness (hypoglycemic coma) and, in exceptional cases, death.
Patients may experience local allergic reactions in the form of redness, swelling or itching at the injection site. These symptoms usually disappear within a few days or weeks. In some cases, these reactions may be due to causes unrelated to insulin, such as skin irritation from the cleansing agent or improper injection administration.
More rarely, generalized allergic reactions occur, in which itching throughout the body, urticaria, angioedema, fever, shortness of breath, decreased blood pressure, tachycardia, and increased sweating may occur. Severe cases of generalized allergic reactions can be life-threatening.
Lipodystrophy may develop at the injection site.
Spontaneous messages:
Cases of edema development were identified, mainly with rapid normalization of blood glucose levels against the background of intensive insulin therapy with initially unsatisfactory glycemic control.
Interaction
Interaction
The severity of the hypoglycemic effect is reduced when administered together with the following drugs: oral contraceptives, glucocorticosteroids, iodine-containing thyroid hormones, danazol, beta2-adrenergic agonists (for example, rigodrine, salbutamol, terbutaline), thiazide diuretics, chlorprothixene, diazoxide, isoniazid, nicotinic acid, phenothiazine derivatives.
The severity of the hypoglycemic effect increases when administered together with the following drugs: beta-blockers, ethanol and ethanol-containing drugs, anabolic steroids, fenfluramine. guanethidine, tetracyclines, oral hypoglycemic drugs, salicylates (for example, acetylsalicylic acid), sulfonamide antibiotics. some antidepressants (monoamine oxidase inhibitors, serotonin reuptake inhibitors), angiotensin-converting enzyme inhibitors (captopril, enapril), octreotide, angiotensin II receptor antagonists.
If you need to use other medications in addition to insulin, you should consult your doctor.
Overdose
Overdose
An overdose is accompanied by the development of symptoms of hypoglycemia: lethargy, increased sweating, hunger, tremor, tachycardia, headache, dizziness, blurred vision, vomiting, confusion.
Mild hypoglycemic episodes are relieved by oral glucose or sugar-containing foods. Correction of moderately severe hypoglycemia can be achieved with intramuscular or subcutaneous administration of glucagon followed by oral carbohydrate once the patient’s condition has stabilized. For patients who do not respond to glucagon, a glucose solution is administered intravenously.
If the patient is in a comatose state, then glucagon should be administered intramuscularly or subcutaneously. In the absence of glucagon or if there is no reaction to its administration, it is necessary to administer a dextrose solution intravenously. Immediately after regaining consciousness, the patient should be given food rich in carbohydrates.
Further maintenance intake of carbohydrates and monitoring of the patient may be required, as relapse of hypoglycemia may occur.
The attending physician must be informed about hypoglycemia.
Storage conditions
Storage conditions
In a place protected from light, at a temperature of 2-8°C; do not freeze.
The drug in use should be stored at room temperature from 15° to 25°C; Protect from direct sunlight and heat.
Shelf life
Shelf life
3 years. Do not use after expiration date.
Manufacturer
Manufacturer
Lilly France, France
Additional information
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | In the dark place at 2-8°C; do not freeze. |
| Manufacturer | Lilly France, France |
| Medication form | solution |
| Brand | Lilly France |
Related products
Buy Humalog QuickPen syringe pen cartridges, 100 me/ml 3 ml QuickPen syringe pen cartridges 5 pcs with delivery to USA, UK, Europe and over 120 other countries.