No products in the cart.
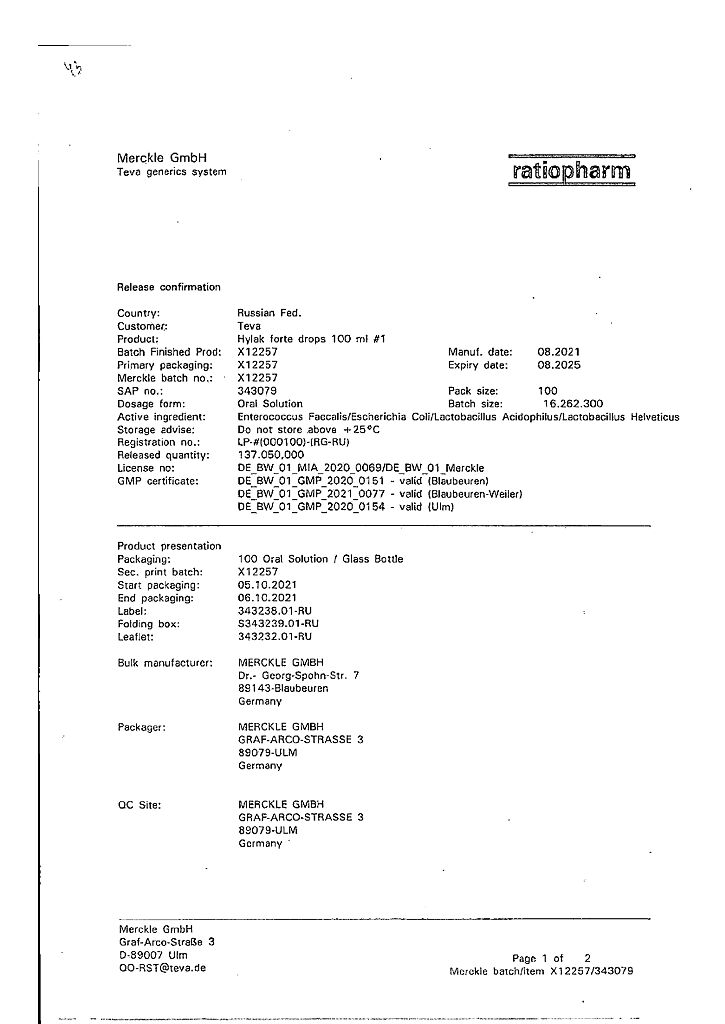
Hilac forte, drops 100 ml
€19.91 €17.25
Out of stock
(E-mail when Stock is available)
Description
Pharmacotherapeutic group: anti-diarrheal medicine
Code. ATX: A07FA
Pharmacological action:
The natural physiological intestinal microflora may be significantly impaired by external influences such as antibiotic therapy, radiation, gastric surgery, or due to unsuitable or unaccustomed diet, water changes, climate change and other conditions.
Hilac forte regulates the balance of the intestinal microflora and normalizes its composition. Due to the composition of Hilac forte contains products of metabolism of normal microflora, the drug promotes the restoration of normal intestinal microflora in a biological way and helps to maintain physiological and biological functions of the intestinal mucosa.
The biosynthetic lactic acid and its buffer salts contained in Hilac forte restore normal acidity in the gastrointestinal tract, regardless of whether the patient has excessive or reduced acidity.
There is evidence that Hilac forte improves protective functions of the body by stimulating the immune response.
When using Hilac forte it is observed accelerated excretion of Salmonella in infants after salmonellosis enteritis due to stimulation of growth of acidophilic anaerobic intestinal flora and its subsequent antagonistic effect on Salmonella.
Indications
Indications
– disruption of the physiological flora of the small and large intestines (during and after treatment with antibiotics or sulfonamides, radiation therapy);
– digestive insufficiency syndrome, dyspepsia;
– diarrhea, flatulence, constipation;
– gastroenteritis, colitis; senile intestinal syndrome (chronic, atrophic gastroenteritis);
– disorders of the gastrointestinal tract caused by climate change;
– hypo- and anacid states;
– enterogenous diseases of the gallbladder and liver;
– allergic skin diseases (urticaria, endogenously caused chronic eczema);
– salmonellosis in the convalescence stage (including in infants).
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: antidiarrheal agent
Code. ATX: A07FA
Pharmacological action:
The natural physiological intestinal microflora can be significantly disrupted by external influences such as antibiotic therapy, radiation, gastric surgery, as well as due to inappropriate or unusual nutrition, changes in water, climate change and other conditions.
Hilak forte regulates the balance of intestinal microflora and normalizes its composition. Due to the content of metabolic products of normal microflora in Hilak Forte, the drug helps restore normal intestinal microflora biologically and allows you to preserve the physiological and biological functions of the intestinal mucosa.
The biosynthetic lactic acid and its buffer salts included in Hilak Forte restore normal acidity in the gastrointestinal tract, regardless of whether the patient suffers from high or low acidity.
Against the background of accelerating the development of normal intestinal symbionts, under the influence of Hilak Forte, the natural synthesis of vitamins B and K is normalized. The short-chain volatile fatty acids contained in Hilak Forte ensure the restoration of damaged intestinal microflora in infectious diseases of the gastrointestinal tract, stimulate the regeneration of epithelial cells of the intestinal wall, and restore the disturbed water-electrolyte balance in the intestinal lumen.
There is evidence that Hilak forte enhances the body’s protective functions by stimulating the immune response.
When using Hilak Forte, there is an acceleration of the elimination of Salmonella in infants after Salmonella enteritis, which is due to stimulation of the growth of acidophilic anaerobic intestinal flora and its subsequent antagonistic effect on Salmonella.
Special instructions
Special instructions
It is not recommended to take this drug simultaneously with milk and dairy products.
Impact on the ability to drive vehicles and machinery
The drug does not affect the ability to drive or engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Metabolites of bacteria Escherichia coli, Enterococcus faecalis, Lactobacillus acidophilus, Lactobacillus
Composition
Composition
100 ml of the drug contains: active ingredients:
Aqueous substrate of metabolic products
Escherichia coli DSM 4087* – 24.9481 g
Aqueous substrate of metabolic products
Enterococcus faecalis DSM 4086* – 12.4741 g
Aqueous substrate of metabolic products
Lactobacillus acidophilus DSM 4149* – 12.4741 g
Aqueous substrate of metabolic products
Lactobacillus helveticus DSM 4183* – 49.8960 g;
* – substrates contain lactose
excipients: disodium phosphate heptahydrate 1.1590 g, dipotassium phosphate 1.2650 g, lactic acid 2.5000-6.2500 g, phosphoric acid, concentrated 0.3520 g, potassium sorbate 0.1135 g, citric acid monohydrate 0.0322 g, water up to 100 ml.
Pregnancy
Pregnancy
The use of Hilak Forte during pregnancy and lactation is considered safe. However, the decision to prescribe the drug is made by the attending physician.
Contraindications
Contraindications
– hypersensitivity to the components of the drug;
– lactase/isomaltase deficiency, lactose intolerance, glucose-galactose malabsorption.
Side Effects
Side Effects
Hilak Forte is well tolerated by patients of any age.
No side effects have been observed to date.
Allergic reactions are possible (skin rash, itching, urticaria); constipation, diarrhea.
Interaction
Interaction
Under the influence of antacid drugs, it is possible to neutralize lactic acid, which is part of the drug Hilak Forte.
Overdose
Overdose
No special measures are required other than routine medical supervision.
Storage conditions
Storage conditions
At a temperature not higher than 25 0C.
Keep out of the reach of children!
Shelf life
Shelf life
Bottles – 4 years.
After opening the bottle – 6 weeks.
Manufacturer
Manufacturer
Merkle GmbH, Germany
Additional information
| Shelf life | Vials – 4 years. After opening the bottle – 6 weeks. |
|---|---|
| Conditions of storage | Temperature not exceeding 25 0C. Store out of the reach of children! |
| Manufacturer | Merkle GmbH, Germany |
| Medication form | oral drops |
| Brand | Merkle GmbH |
Related products
Buy Hilac forte, drops 100 ml with delivery to USA, UK, Europe and over 120 other countries.