No products in the cart.
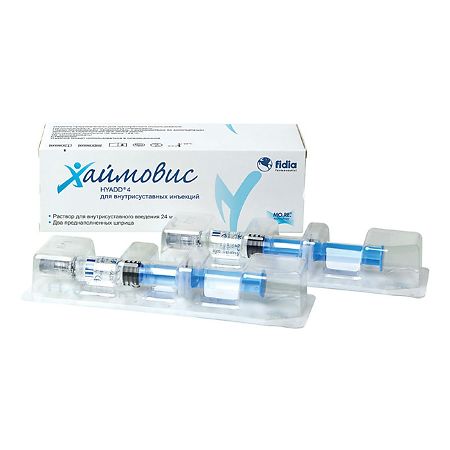
Haimovis 24mg/3ml syringe, 2 pcs.
€1.00
Out of stock
(E-mail when Stock is available)
Description
Haimovis® is a hydrogel that is produced according to Good Manufacturing Practice (GMP) and contains HYADD®4, a new molecule patented by Fidia, at a concentration of 8 mg/ml. HYADD®4 is a new hyaluronic acid derivative: partial hyaluronic acid hexadecylamide (partial due to the very low level of hyaluronic acid modification, at approximately 3% molar concentration to maintain biocompatibility).
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
Directions for use
Directions for use
Synopsis
Synopsis
Contraindications
Contraindications
Side effects
Side effects
Local pain, swelling, fever, and redness may rarely occur at the injection site; such symptoms are usually mild and passable.
More severe inflammatory reactions have been reported, with precipitation of sodium pyrophosphate crystals when sodium hyaluronate solutions are injected intra-articularly, although no relationship has been identified.
If general precautions are not followed and the injection site is not aseptically treated, septic arthritis may occur in rare cases, as for any intra-articular treatment.
Similarities
Similarities
Additional information
| Shelf life | 36 months. |
|---|---|
| Conditions of storage | – Store the medical product in the original package at 2 to 25°C. – Do not freeze. |
| Manufacturer | Fidia Farmaceutici S.p.A., Italy |
| Medication form | solution for injection |
| Brand | Fidia Farmaceutici S.p.A. |
Other forms…
Related products
Buy Haimovis 24mg/3ml syringe, 2 pcs. with delivery to USA, UK, Europe and over 120 other countries.