No products in the cart.
Haileflox, 500 mg 5 pcs
€9.77 €8.55
Out of stock
(E-mail when Stock is available)
Description
Pharmacological action – broad spectrum antibacterial.
Pharmacodynamics
Levofloxacin is a synthetic fluoroquinolone of broad spectrum. It inhibits DNA-enzyme (topoisomerase II) and topoisomerase IV, breaks supercoiling and cross-linking of DNA breaks, inhibits DNA synthesis, causes deep morphological changes in cytoplasm, cell wall and membranes of sensitive microorganisms.
Levofloxacin is effective against most strains of the following microorganisms:
Aerobic Gram-positive microorganisms – Corynebacterium diphtheriae, Enterococcus spp. (including Enterococcus faecalis), Listeria monocytogenes, Staphylococcus spp. (leukotoxin-containing and coagulase-negative methicillin-sensitive/moderately sensitive strains, including methicillin-sensitive strains of Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus spp. Streptococcus agalactiae, Streptococcus pyogenes, penicillin-sensitive/moderately sensitive/resistant strains of Streptococcus pneumoniae, penicillin-sensitive/resistant strains of Streptococcus viridans);
aerobic gram-negative microorganisms – Acinetobacter spp. (including Acinetobacter baumanii), Actinobacillus actinomycetemcomitans, Citrobacter freundii, Eikenella corrodens, Enterobacter spp. (including Enterobacter aerogenes, Enterobacter agglomerans, Enterobacter cloacae), Escherichia coli, Gardnerella vaginalis, Haemophilus spp. (including Haemophilus ducreyi, Haemophilus parainfluenzae, ampicillin-sensitive/resistant strains of Haemophilus influenzae), Helicobacter pylori, Klebsiella spp. (including Klebsiella oxytoca, Klebsiella pneumoniae), Moraxela catarrhalis (beta-lactamase producing and non-producing strains), Morganella morganii, Neisseria spp. Neisseria meningitidis producing and non-producing strains of Neisseria gonorroeae), Pasteurella spp. (including Pasteurella conis, Pasteurella dagmatis, Pasteurella multocida), Proteus spp. (including Proteus mirabilis, Proteus vulgaris), Providencia spp. (including Providencia rettgeri, Providencia stuartii), Pseudomonas spp. (including Pseudomonas aeruginosa), Serratia spp. (including Serratia marcescens), Salmonella spp, Clostridium perfringens, Fusobacterium spp., Peptostreptococcus spp., Propionibacterum spp., Veilonella spp.;
other microorganisms – Bartonella spp, Chlamydia spp. (including Chlamydia pneumoniae, Chlamydia psittaci, Chlamydia trachomatis), Legionella pneumophila, Mycobacterium spp. (including Mycobacterium leprae, Mycobacterium tuberculosis), Mycoplasma spp. (including Mycoplasma hominis, Mycoplasma pneumoniae), Rickettsia spp, Ureaplasma urealyticum.
Resistant microorganisms:
aerobic gram-positive microorganisms – Corynebacterium jeikeium, Staphylococcus spp. (coagulase-negative methicillin-resistant strains, incl. methicillin-resistant strains of Staphylococcus aureus);
aerobic Gram-negative microorganisms – Alcaligenes xylosoxidans;
other microorganisms – Mycobacterium avium.
Pharmacokinetics
Absorption: after oral administration levofloxacin is quickly and almost completely absorbed from the gastrointestinal tract. Food has little effect on the speed and completeness of absorption. Bioavailability is 99%. Cmax in plasma is reached after 1-2 hours and for levofloxacin 250, 500 and 750 mg doses it is 2.8; 5.2 and 8 mcg/ml, respectively.
Distribution: After a single or multiple dose the amount of the drug absorbed is directly proportional to the dose taken. Equilibrium concentration (CSS) in plasma is reached after 48 hours. Mean volume of distribution (Vd) of levofloxacin varies from 74 to 112 l. Binding to plasma proteins is 30-40%. It penetrates well into cells, tissues, organs and secretions: lungs, bronchial mucosa, sputum, alveolar macrophages (concentration in lung tissue is 2-5 times higher than in plasma), urogenital organs, polymorphonuclear leukocytes.
Metabolism: levofloxacin undergoes limited metabolism in the liver (oxidation and/or deacetylation).
Elimation: is excreted from the body mainly by the kidneys through glomerular filtration and tubular secretion. T1/2 of levofloxacin is 6-8 hours. Less than 5% of the administered dose is excreted as desmethyl- and N-oxide-metabolites. Unchanged by the kidneys 70% of the oral dose is excreted within 24 hours and 87% within 48 hours. 4% of the dose taken orally is excreted in the intestine within 72 hours.
Indications
Indications
Infectious and inflammatory diseases of mild to moderate severity, caused by microorganisms sensitive to the drug:
– lower respiratory tract infections (pneumonia, exacerbation of chronic bronchitis);
– acute bacterial sinusitis;
– infections of the urinary tract and kidneys (including acute pyelonephritis);
– skin and soft tissue infections (suppurative atheromas, abscesses, furuncles);
– chronic bacterial prostatitis;
– intra-abdominal infection (in combination with antibacterial drugs acting on anaerobic microflora);
– tuberculosis (as part of complex therapy of drug-resistant forms).
Active ingredient
Active ingredient
Composition
Composition
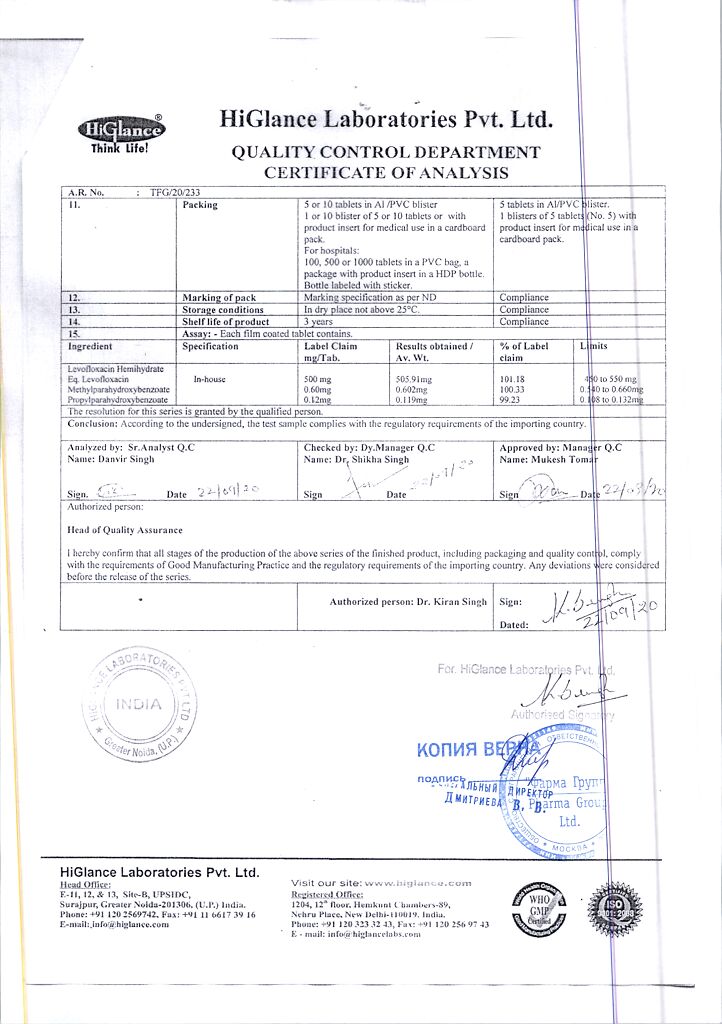
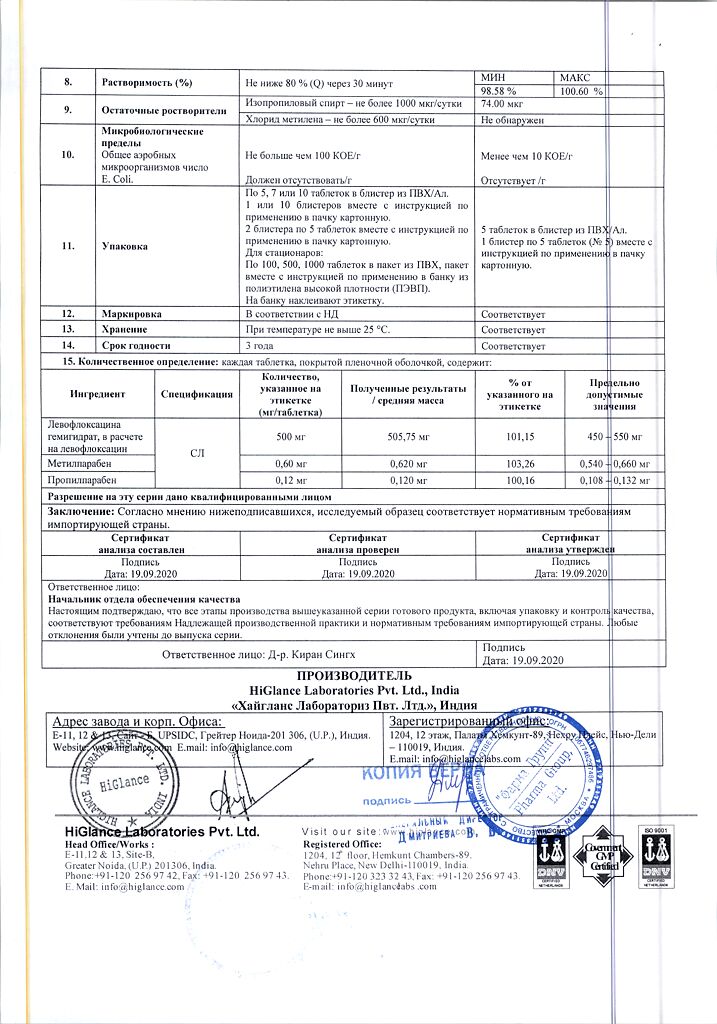
1 tablet contains levofloxacin 500 mg
How to take, the dosage
How to take, the dosage
Haileflox is taken orally, before or between meals, without chewing, with plenty of water.
In adults with normal renal function (Cl creatinine >50 ml/min), use according to the regimens presented in the table:
Hospital pneumonia 750 mg once daily. 7-14 days.
Out-of-hospital pneumonia 500 mg 1-2 times/day. 7-14 days; 750 mg once daily. 5* days.
The exacerbation of chronic bronchitis 500 mg 1 time/day. 7 days.
Acute bacterial sinusitis 500 mg 1 time/day. 10-14 days; 750 mg 1 time/day. 5 days.
Uncomplicated urinary tract infections 250 mg once daily.
Complicated urinary tract infections, including acute pyelonephritis 250 mg 1 time/day. 10** days; 750 mg 1 time/day.
Uncomplicated infections of the skin and subcutaneous tissues 500 mg 1 time/day. 7-10 days.
Complicated skin and subcutaneous tissue infections 750 mg once daily. 7-14 days.
Chronic bacterial prostatitis 500 mg once daily. 28 days.
Intra-abdominal infection (in combination with antibacterials acting on anaerobic microflora) 500 mg once daily. 7-14 days.
Tuberculosis (as part of complex therapy of drug-resistant forms) 750 mg once daily. up to 3 months
* This regimen is indicated for treatment of community-acquired pneumonia caused by Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, Mycoplasma pneumoniae, Chlamydia pneumoniae.
** This regimen is indicated for the treatment of urinary tract infections caused by Enterococcus faecalis, Enterococcus cloacae, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, and acute pyelonephritis caused by Escherichia coli.
*** This regimen is indicated for the treatment of urinary tract infections caused by Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and acute pyelonephritis caused by Escherichia coli, including cases with associated bacteremia.
Interaction
Interaction
Levofloxacin increases the half-life of cyclosporine.
The effect of levofloxacin is reduced by drugs that inhibit intestinal motility, sucralfate, aluminum- or magnesium-containing antacids and iron preparations.
Theophylline and NSAIDs when used concomitantly with levofloxacin increase the risk of seizures in predisposed patients, and GCS increase the risk of tendon rupture.
The concomitant use of levofloxacin with hypoglycemic drugs may result in changes in blood glucose levels, including hyperglycemia and hypoglycemia.
Levofloxacin increases the effect of warfarin.
Cimetidine and drugs that block tubular secretion slow the excretion of levofloxacin.
Special Instructions
Special Instructions
After normalization of body temperature, it is recommended to continue treatment for at least 48-72 h. Levofloxacin is taken at least 2 hours before or 2 hours after taking magnesium/aluminum antacids or sucralfate, or other drugs containing calcium, iron or zinc.
Due to possible photosensitization during treatment and within 5 days after treatment with levofloxacin it is necessary to avoid sunlight and artificial UV exposure. If phototoxicity develops, treatment with the drug should be discontinued. If signs of tendinitis and pseudomembranous colitis appear, levofloxacin should be immediately discontinued. It should be borne in mind that patients with a history of brain damage (stroke, severe trauma) may develop convulsions. In patients with glucose-6-phosphate dehydrogenase deficiency there is a possible risk of hemolytic reactions.
In diabetic patients during treatment with levofloxacin blood glucose levels should be carefully monitored. During concomitant use of levofloxacin and warfarin monitoring of PV, INR or other anticoagulation tests, as well as monitoring of bleeding signs is indicated. During levofloxacin administration the patient’s ability to concentrate and speed of psychomotor reactions may be impaired.
In this regard it is necessary to exercise caution when driving motor transport and engaging in other potentially dangerous activities.After normalization of body temperature it is recommended to continue treatment for at least 48-72 hours. Levofloxacin is taken at least 2 hours before or 2 hours after taking magnesium/aluminum antacids or sucralfate, or other drugs containing calcium, iron or zinc.
Due to possible photosensitization during treatment and within 5 days after treatment with levofloxacin it is necessary to avoid sunlight and artificial UV exposure. In case of development of phototoxicity the drug therapy should be stopped.
In case of signs of tendinitis and pseudomembranous colitis levofloxacin should be immediately discontinued. It should be borne in mind that patients with a history of brain damage (stroke, severe trauma) may develop convulsions. In glucose-6-phosphate dehydrogenase deficiency there may be a risk of hemolytic reactions. In diabetic patients during treatment with levofloxacin blood glucose levels should be carefully monitored.
In concomitant use of levofloxacin and warfarin, monitoring of PV, INR or other anticoagulation tests and monitoring of bleeding signs are indicated.
The patient’s ability to concentrate and quick psychomotor reactions may be impaired during levofloxacin administration. In this regard, caution should be exercised when driving motor transport and engaging in other potentially dangerous activities.
Contraindications
Contraindications
With caution – advanced age (high probability of concomitant decreased renal function), glucose-6-phosphate dehydrogenase deficiency.
Side effects
Side effects
Nervous system disorders: headache, dizziness, weakness, drowsiness, insomnia, tremor, anxiety, paresthesias, fear, hallucinations, confusion, depression, motor disorders, seizures.
Sensory organs: disorders of vision, hearing, smell, taste and tactile sensitivity.
Systemic reactions: decreased blood pressure, vascular collapse, tachycardia, prolongation of QT interval, atrial fibrillation.
Digestive system disorders: nausea, vomiting, diarrhea (including blood), digestive disorders, decreased appetite, abdominal pain, pseudomembranous colitis; increased liver transaminases activity, hyperbilirubinemia, hepatitis, dysbiosis.
Metabolic disorders: hypoglycemia (increased appetite, increased sweating, shivering, nervousness).
Muscular system: arthralgia, muscle weakness, myalgia, rhabdomyolysis, tendon rupture, tendinitis.
Urinary system disorders: hypercreatininemia, interstitial nephritis, acute renal failure.
Hematopoietic organs: eosinophilia, hemolytic anemia, leukopenia, neutropenia, agranulocytosis, thrombocytopenia, pancytopenia, hemorrhages.
Allergic reactions: itching and hyperemia of the skin, swelling of the skin and mucous membranes, urticaria, erythema malignant exudative (Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell syndrome), bronchospasm, choking, anaphylactic shock, allergic pneumonitis, vasculitis.
Others: photosensitization, asthenia, exacerbation of porphyria, persistent fever, development of superinfection.
Overdose
Overdose
Symptoms: nausea, erosive lesions of gastrointestinal mucous membranes, prolonged QT interval, confusion, dizziness, seizures.
Treatment: gastric lavage, if necessary – symptomatic therapy. There is no specific antidote, dialysis is ineffective.
Similarities
Similarities
Additional information
| Weight | 0.014 kg |
|---|---|
| Shelf life | 3 years |
| Conditions of storage | In a dry, light-protected place at 8-25 °C |
| Manufacturer | Hyglans Laboratories Pvt. Ltd, India |
| Medication form | pills |
| Brand | Hyglans Laboratories Pvt. Ltd |
Related products
Buy Haileflox, 500 mg 5 pcs with delivery to USA, UK, Europe and over 120 other countries.