No products in the cart.
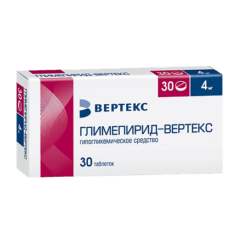
Glimepiride-Vertex, tablets 3 mg 30 pcs
€11.52 €10.08
Description
Glimepiride is an oral hypoglycemic drug – a sulfonylurea derivative of new (third) generation. Glimepiride acts mainly by stimulating the secretion and release of insulin from the beta cells of the pancreas (pancreatic action). As with other sulfonylurea derivatives, this effect is based on increasing the response of pancreatic beta cells to physiologic glucose stimulation, and the amount of insulin secreted is significantly lower than with traditional sulfonylurea derivatives.
The least stimulating effect of glimepiride on insulin secretion also provides a lower risk of hypoglycemia. In addition, glimepiride has extrapancreatic action – the ability to improve the sensitivity of peripheral tissues (muscle, fat) to the action of its own insulin, reduce insulin uptake by the liver; it inhibits the production of glucose in the liver. Glimepiride selectively inhibits cyclooxygenase and reduces the conversion of arachidonic acid to thromboxane A2, which promotes platelet aggregation, thus having an antithrombotic effect.
Glimepiride promotes normalization of lipids, reduces the level of low aldehyde in blood, which leads to a significant decrease in lipid peroxidation, this promotes the antiatherogenic effect of the drug.
Glimepiride increases the level of endogenous α-tocopherol, catalase activity, glutathione peroxidase and superoxide dismutase which helps to decrease the intensity of oxidative stress in patient’s organism that is constantly present in type 2 diabetes.
Indications
Indications
The drug is indicated for the treatment of type 2 diabetes mellitus when previously prescribed diet and physical activity are ineffective.
If monotherapy with Glimepiride is ineffective, it can be used in combination therapy with metformin or insulin.
Pharmacological effect
Pharmacological effect
Glimepiride is a hypoglycemic drug for oral use – a new (third) generation sulfonylurea derivative. Glimepiride acts primarily by stimulating the secretion and release of insulin from the beta cells of the pancreas (pancreatic action). As with other sulfonylureas, this effect is based on an increase in the response of pancreatic beta cells to physiological stimulation by glucose, while the amount of insulin secreted is significantly less than with traditional sulfonylurea drugs.
The least stimulating effect of glimepiride on insulin secretion also provides a lower risk of developing hypoglycemia. In addition to this, glimepiride has an extrapancreatic effect – the ability to improve the sensitivity of peripheral tissues (muscle, fat) to the action of its own insulin, reduce the absorption of insulin by the liver; inhibits glucose production in the liver. Glimepiride selectively inhibits cyclooxygenase and reduces the conversion of arachidonic acid to thromboxane A2, which promotes platelet aggregation, thus exerting an antithrombotic effect.
Glimepiride helps normalize lipid levels, reduces the level of minor aldehyde in the blood, which leads to a significant reduction in lipid peroxidation, which contributes to the antiatherogenic effect of the drug.
Glimepiride increases the level of endogenous α-tocopherol, the activity of catalase, glutathione peroxidase and superoxide dismutase, which helps reduce the severity of oxidative stress in the patient’s body, which is constantly present in type 2 diabetes.
Special instructions
Special instructions
Glimepiride should be taken at the recommended doses and at the prescribed times. Errors in use of the drug, such as missing doses, should never be corrected by subsequent administration of a higher dose. The doctor and the patient should discuss in advance the measures that should be taken in case of such errors (for example, skipping a drug dose or meal) or in situations where it is impossible to take the next dose of the drug at the prescribed time. The patient should immediately inform the doctor if the dose of the drug is too high.
If a patient develops a hypoglycemic reaction when taking 1 mg of glimepiride per day, this indicates that this patient can normalize blood glucose levels with diet alone.
When compensation for type 2 diabetes mellitus is achieved, insulin sensitivity increases. In this regard, the need for Glimepiride may decrease during treatment. To avoid the development of hypoglycemia, it is necessary to temporarily reduce the dose or discontinue Glimepiride. Dose adjustment should also be carried out when the patient’s body weight changes, when his lifestyle changes, or when other factors appear that increase the risk of developing hypo- or hyperglycemia. Adequate diet, regular and sufficient exercise and, if necessary, weight loss are as important for achieving optimal blood glucose control as regular use of glimepiride.
Clinical symptoms of hyperglycemia (insufficient reduction of blood glucose levels) are: increased frequency of urination, extreme thirst, dry mouth and dry skin. In the first weeks of treatment, the risk of developing hypoglycemia may increase, which requires particularly careful monitoring of the patient. During treatment with Glimepiride, hypoglycemia may develop if you eat irregularly or skip meals.
Its possible symptoms are: headache, hunger, nausea, vomiting, feeling tired, drowsiness, sleep disturbances, anxiety, aggressiveness, problems with concentration, attention and reaction, depression, confusion, speech and visual disturbances, aphasia, tremor, paresis, sensory disturbances, dizziness, delirium, cerebral spasms, confusion or loss of consciousness, including coma, shallow breathing, bradycardia. In addition, as a result of the adrenergic feedback mechanism, sweating, anxiety, tachycardia, increased blood pressure, angina pectoris and cardiac arrhythmias may occur. Factors contributing to the development of hypoglycemia include:
reluctance or (especially in old age) insufficient ability of the patient to cooperate with the doctor;
inadequate, irregular nutrition, skipping meals, fasting, changes in the usual diet;
imbalance between physical activity and carbohydrate intake;
drinking alcohol, especially in combination with skipping meals;
renal dysfunction;
severe liver dysfunction;
overdose of Glimepiride;
some uncompensated diseases of the endocrine system that affect carbohydrate metabolism (for example, thyroid dysfunction, pituitary insufficiency or adrenal insufficiency);
simultaneous use of drugs that enhance the effect of Glimepiride.
The doctor should be informed about the above factors and about episodes of hypoglycemia, since they require particularly strict monitoring of the patient. If there are such factors that increase the risk of hypoglycemia, the dose of Glimepiride or the entire treatment regimen should be adjusted. This must also be done in the case of an intercurrent illness or a change in the patient’s lifestyle.
Symptoms of hypoglycemia may be smoothed out or completely absent in elderly patients, in patients suffering from autonomic neuropathy or receiving concomitant treatment with beta-blockers, clonidine, reserpine, guanethidine or other sympatholytic agents. Hypoglycemia can almost always be quickly reversed by immediate intake of carbohydrates (glucose or sugar, for example in the form of a sugar cube, sweet fruit juice or tea). In this regard, the patient should always have at least 20 g of glucose (4 lumps of sugar) with him. Sweeteners are ineffective in treating hypoglycemia.
From the experience of using other sulfonylurea drugs, it is known that, despite the initial success in relieving hypoglycemia, its recurrence is possible. In this regard, continuous and careful monitoring of the patient is necessary. Severe hypoglycemia requires immediate treatment under medical supervision, and under certain circumstances, hospitalization of the patient.
If a patient suffering from diabetes is treated by different doctors (for example, while staying in the hospital after an accident, when sick on the weekend), he must inform them about his disease and previous treatment.
During treatment with Glimepiride, regular monitoring of liver function and peripheral blood patterns (especially the number of leukocytes and platelets) is required.
In stressful situations (for example, trauma, surgery, infectious diseases accompanied by fever), it may be necessary to temporarily transfer the patient to insulin therapy.
There is no experience with the use of Glimepiride in patients with severely impaired liver and kidney function or patients on hemodialysis. Patients with severely impaired renal and liver function are advised to switch to insulin therapy.
During treatment with Glimepiride, regular monitoring of the concentration of glucose in the blood, as well as the concentration of glycosylated hemoglobin, is necessary.
At the beginning of treatment, when switching from one drug to another, or when taking Glimepiride irregularly, there may be a decrease in the patient’s concentration and speed of psychomotor reactions due to hypo- or hyperglycemia. This may adversely affect the ability to drive vehicles or operate various machines and mechanisms.
Active ingredient
Active ingredient
Glimepiride
Composition
Composition
One tablet contains:
Active substance
glimepiride – 3 mg
Excipients:
lactose (milk sugar),
microcrystalline cellulose,
pregelatinized starch,
sodium lauryl sulfate,
magnesium stearate.
Contraindications
Contraindications
Diabetes mellitus type 1; diabetic ketoacidosis, diabetic precoma and coma; hyperosmolar coma; hypersensitivity to glimepiride or to any inactive component of the drug, to other sulfonylurea derivatives or to sulfonamide drugs (risk of developing hypersensitivity reactions); severe liver dysfunction; severe renal dysfunction (including patients on hemodialysis); lactose intolerance, lactase deficiency, glucose-galactose malabsorption; children under 18 years of age; glucose-6-phosphate dehydrogenase deficiency; pregnancy and breastfeeding period.
With caution – conditions requiring transfer of the patient to insulin therapy (extensive burns, severe multiple trauma, extensive surgical interventions); adrenal insufficiency; diseases of the thyroid gland (hypothyroidism, thyrotoxicosis); disturbances in the absorption of food and drugs in the gastrointestinal tract, including intestinal obstruction, intestinal paresis; infectious fever; alcoholism; in the first days of treatment (increased risk of developing hypoglycemia); with an increased risk of developing hypoglycemia; for intercurrent diseases during treatment or when changing the patient’s lifestyle (changing diet and meal times, increasing or decreasing physical activity).
Side Effects
Side Effects
Rare:
Metabolism: development of hypoglycemic reactions. These reactions mainly occur soon after taking the drug and are not always easily controlled.
On the part of the visual organs: during treatment (especially at the beginning), a transient decrease in vision may be observed due to changes in the concentration of glucose in the blood.
From the digestive system: increased activity of liver enzymes, cholestasis, jaundice, hepatitis (up to the development of liver failure).
From the hematopoietic system: thrombocytopenia (moderate to severe), leukopenia, hemolytic or aplastic anemia, erythrocytopenia, granulocytopenia, agranulocytosis and pancytopenia.
Sometimes:
From the digestive system: nausea, vomiting, feeling of heaviness or discomfort in the epigastrium, abdominal pain, diarrhea, very rarely leading to cessation of treatment.
Allergic reactions: the appearance of symptoms of urticaria (itching, skin rash). Such reactions are usually moderate, but can progress, accompanied by a drop in blood pressure, dyspnea, and even the development of anaphylactic shock. If symptoms of hives appear, you should consult a doctor immediately. Cross-allergy with other sulfonylurea derivatives, sulfonamides, or similar substances is possible, and the development of allergic vasculitis is also possible.
In exceptional cases:
Other side effects: possible development of photosensitivity, hyponatremia.
Since certain side effects, such as: severe hypoglycemia, serious changes in the blood picture, severe allergic reactions, liver failure, can, under certain circumstances, pose a threat to life, in the event of the development of undesirable or severe reactions, the patient must immediately inform the attending physician about them and in no case continue taking the drug without his recommendation.
Interaction
Interaction
Glimepiride is metabolized by cytochrome P450 2C9 (CYP2C9). When used simultaneously with inducers of the CYP2C9 isoenzyme, for example, rifampicin, the hypoglycemic effect of glimepiride may decrease and the risk of hypoglycemia may increase if they are discontinued without dose adjustment of glimepiride.
When used simultaneously with inhibitors of the CYP2C9 isoenzyme, for example, fluconazole, it is possible to enhance the hypoglycemic effect of glimepiride and increase the risk of hypoglycemia and side effects of glimepiride, and it is also possible to reduce its hypoglycemic effect when they are canceled without adjusting the dose of glimepiride.
An enhanced hypoglycemic effect and the associated possible development of hypoglycemia can be observed with simultaneous use of glimepiride with insulin or other oral hypoglycemic drugs, metformin, angiotensin-converting enzyme (ACE) inhibitors, allopurinol, anabolic steroids and male sex hormones. hormones, chloramphenicol, coumarin derivatives, cyclo-, tro- and isophosphamides, fenfluramine, disopyramide, fibrates, fluoxetine, sympatholytics (guanethidine), monoamine oxidase inhibitors (MAO), miconazole, fluconazole, pentoxifylline (when administered parenterally in high doses), phenylbutazone, azapropazone, oxyphenbutazone, probenecid, quinolones, salicylates and aminosalicylic acid, sulfinpyrazone, some long-acting sulfonides, tetracyclines, tritoqualine. A weakening of the hypoglycemic effect and an associated increase in the concentration of glucose in the blood can be observed with simultaneous use of glimepiride with acetazolamide, barbiturates, glucocorticosteroids, glucagon, laxatives (with long-term use), nicotinic acid (in high doses) and derivatives of nicotinic acid, estrogens and progestogens, phenothiazines, chlorpromazine, phenytoin, rifampicin, thyroid hormones, lithium salts. H2-histamine receptor blockers, clonidine and reserpine, can both potentiate and weaken the hypoglycemic effect of glimepiride.
Under the influence of sympatholytic agents such as beta-blockers, clonidine, guanethidine and reserpine, clinical signs of hypoglycemia may be weakened or absent. While taking glimepiride, an increase or decrease in the effect of coumarin derivatives may be observed. When used simultaneously with drugs that inhibit bone marrow hematopoiesis, the risk of myelosuppression increases. Single or chronic consumption of alcohol can either enhance or weaken the hypoglycemic effect of glimepiride.
Overdose
Overdose
Symptoms:
hypoglycemia (nausea, vomiting and epigastric pain, anxiety, tremors, visual disturbances, coordination problems, drowsiness, coma and convulsions).
Treatment:
if the patient is conscious – induction of vomiting, drinking plenty of fluids, activated charcoal and a laxative. In case of severe overdose, an intravenous bolus of dextrose solution (50 ml of a 40% solution), then an infusion of a 10% solution. It is necessary to constantly monitor the patient, maintain vital functions and control the concentration of glucose in the blood (recurrence of hypoglycemia episodes is possible). Subsequent treatment is symptomatic.
Storage conditions
Storage conditions
At a temperature not higher than 30°C. Keep out of the reach of children.
Manufacturer
Manufacturer
Vertex, Russia
Additional information
| Conditions of storage | At a temperature not exceeding 30 ° C. Keep out of reach of children. |
|---|---|
| Manufacturer | Vertex, Russia |
| Medication form | pills |
| Brand | Vertex |
Other forms…
Related products
Buy Glimepiride-Vertex, tablets 3 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.