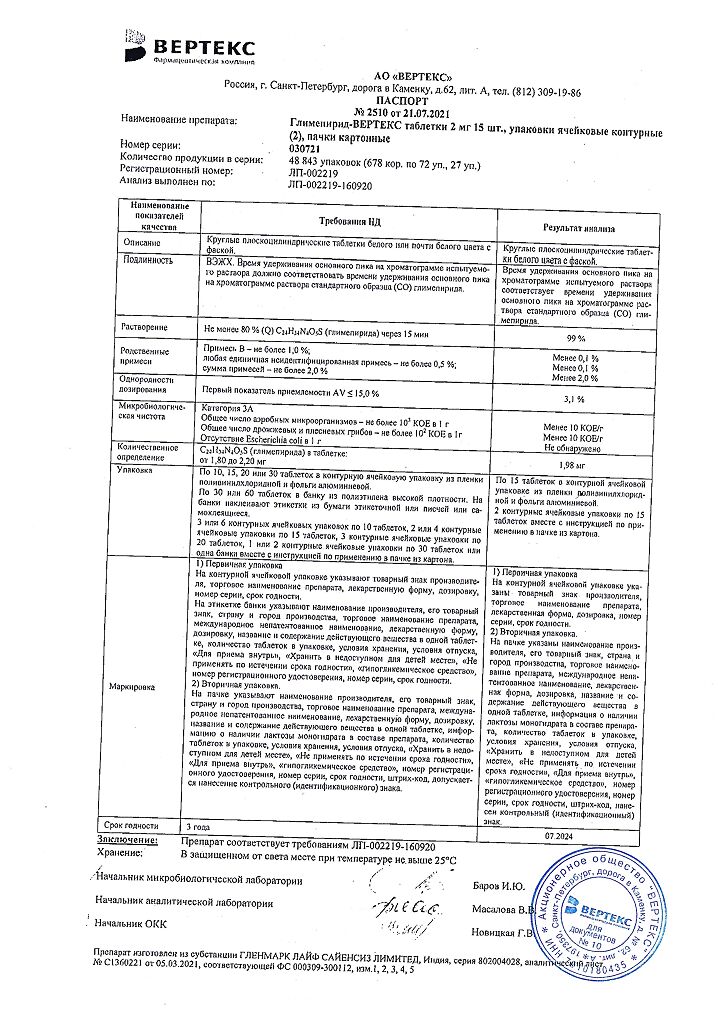
No products in the cart.
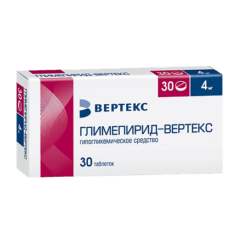
Glimepiride-Vertex, tablets 2 mg 30 pcs
€7.59 €6.64
Description
Glimepiride is an oral hypoglycemic drug – a sulfonylurea derivative of new (third) generation. Glimepiride acts mainly by stimulating secretion and release of insulin from beta-cells of the pancreas (pancreatic action).
As with other sulfonylurea derivatives, this effect is based on an increased response of pancreatic beta cells to physiologic glucose stimulation, with significantly less insulin secreted than with traditional sulfonylurea derivatives. The smallest stimulating effect of glimepiride on insulin secretion also reduces the risk of hypoglycemia. In addition, glimepiride has extrapancreatic action – the ability to improve the sensitivity of peripheral tissues (muscle, fat) to the action of its own insulin, reduce insulin uptake by the liver; it inhibits the production of glucose in the liver.
Glimepiride selectively inhibits cyclooxygenase and reduces the conversion of arachidonic acid to thromboxane A2, which promotes platelet aggregation, thus having an antithrombotic effect.
Glimepiride promotes normalization of lipids, reduces the level of low aldehyde in the blood, which leads to a significant decrease in lipid peroxidation, this promotes the anti-atherogenic effect of the drug.
Glimepiride increases the level of endogenous α-tocopherol, catalase activity, glutathione peroxidase and superoxide dismutase that helps to decrease the severity of oxidative stress in patient’s body which is constantly present in type 2 diabetes.
Indications
Indications
Diabetes mellitus type 2.
Pharmacological effect
Pharmacological effect
Glimepiride is a hypoglycemic drug for oral use – a new (third) generation sulfonylurea derivative. Glimepiride acts primarily by stimulating the secretion and release of insulin from the beta cells of the pancreas (pancreatic action).
As with other sulfonylureas, this effect is based on an increase in the response of pancreatic beta cells to physiological stimulation by glucose, while the amount of insulin secreted is significantly less than with traditional sulfonylurea drugs. The least stimulating effect of glimepiride on insulin secretion also provides a lower risk of developing hypoglycemia. In addition to this, glimepiride has an extrapancreatic effect – the ability to improve the sensitivity of peripheral tissues (muscle, fat) to the action of its own insulin, reduce the absorption of insulin by the liver; inhibits glucose production in the liver.
Glimepiride selectively inhibits cyclooxygenase and reduces the conversion of arachidonic acid to thromboxane A2, which promotes platelet aggregation, thus exerting an antithrombotic effect.
Glimepiride helps normalize lipid levels, reduces the level of minor aldehyde in the blood, which leads to a significant reduction in lipid peroxidation, which contributes to the antiatherogenic effect of the drug.
Glimepiride increases the level of endogenous α-tocopherol, the activity of catalase, glutathione peroxidase and superoxide dismutase, which helps reduce the severity of oxidative stress in the patient’s body, which is constantly present in type 2 diabetes.
Active ingredient
Active ingredient
Glimepiride
Composition
Composition
Active ingredient:
glimepiride 2 mg;
Excipients:
lactose monohydrate 150.80 mg,
corn starch 4.66 mg,
sodium carboxymethyl starch 10.00 mg,
povidone 6.00 mg,
polysorbate 1.34 mg,
talc 2.00 mg,
magnesium stearate 1.20 mg,
iron dye (E 172) 0.30 mg
Contraindications
Contraindications
Hypersensitivity, type 1 diabetes mellitus, diabetic ketoacidosis, diabetic precoma and coma, severe liver and kidney dysfunction, leukopenia, pregnancy, breastfeeding.
Side Effects
Side Effects
From the cardiovascular system and blood (hematopoiesis, hemostasis): rarely – decreased blood pressure, thrombocytopenia, leukopenia, granulocytopenia, agranulocytosis, erythropenia, pancytopenia, hemolytic and aplastic anemia.
From the nervous system and sensory organs: dizziness, headache, transient visual impairment.
From the gastrointestinal tract: nausea, vomiting, abdominal pain, feeling of heaviness in the epigastric region, diarrhea, intrahepatic cholestasis.
Metabolism: hypoglycemia.
Other: increased levels of transaminases, hyponatremia, allergic skin reactions, porphyria cutanea tarda, asthenia; rarely – shortness of breath, hepatitis, allergic vasculitis, photosensitivity.
Interaction
Interaction
Strengthening the hypoglycemic effect of glimepiride is possible with simultaneous use with insulin or other hypoglycemic drugs, ACE inhibitors, allopurinol, anabolic steroids and male sex hormones, chloramphenicol, coumarin derivatives, cyclophosphamide, disopyramide, fenfluramine, pheniramidol, fibrates, fluoxetine, guanethidine, isophosphamides, MAO inhibitors, miconazole, PAS, pentoxifylline (when administered in high doses), phenylbutazone, azapropazone, oxyphenbutazone, probenecid, quinolones, salicylates, sulfinpyrazone, sulfonamides, tetracyclines.
Weakening of the hypoglycemic effect of glimepiride is possible with simultaneous use with acetazolamide, barbiturates, corticosteroids, diazoxide, diuretics, epinephrine (adrenaline) and other sympathomimetics, glucagon, laxatives (after long-term use), nicotinic acid (in high doses), estrogens and progestogens, phenothiazine, phenytoin, rifampicin, thyroid hormones.
When used simultaneously, histamine H2 receptor blockers, clonidine and reserpine can both potentiate and reduce the hypoglycemic effect of glimepiride.
The use of glimepiride may enhance or weaken the effect of coumarin derivatives.
Ethanol may enhance or weaken the hypoglycemic effect of glimepiride.
Overdose
Overdose
Symptoms
: hypoglycemia (nausea, vomiting and epigastric pain, anxiety, tremors, visual disturbances, coordination problems, drowsiness, coma and convulsions).
Treatment:
if the patient is conscious – induction of vomiting, drinking plenty of fluids, activated charcoal and a laxative. In case of severe overdose, an intravenous bolus of dextrose solution (50 ml of a 40% solution), then an infusion of a 10% solution. It is necessary to constantly monitor the patient, maintain vital functions and control the concentration of glucose in the blood (recurrence of hypoglycemia episodes is possible). Subsequent treatment is symptomatic.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 25 ° C, in a tightly closed container.
Shelf life
Shelf life
5 years
Manufacturer
Manufacturer
Vertex, Russia
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | In a light-protected place at a temperature not exceeding 25 °C, in a tightly closed container. |
| Manufacturer | Vertex, Russia |
| Medication form | pills |
| Brand | Vertex |
Other forms…
Related products
Buy Glimepiride-Vertex, tablets 2 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.