No products in the cart.
Gestarella, 75 mcg+20 mcg 21 pcs
€13.56 €11.86
EAN: 8594739205291
SKU: 250447
Categories: Contraceptive, Gynecology and Obstetrics, Hormonal, Medicine
Description
Contraception
Oral contraception.
Active ingredient
Active ingredient
Composition
Composition
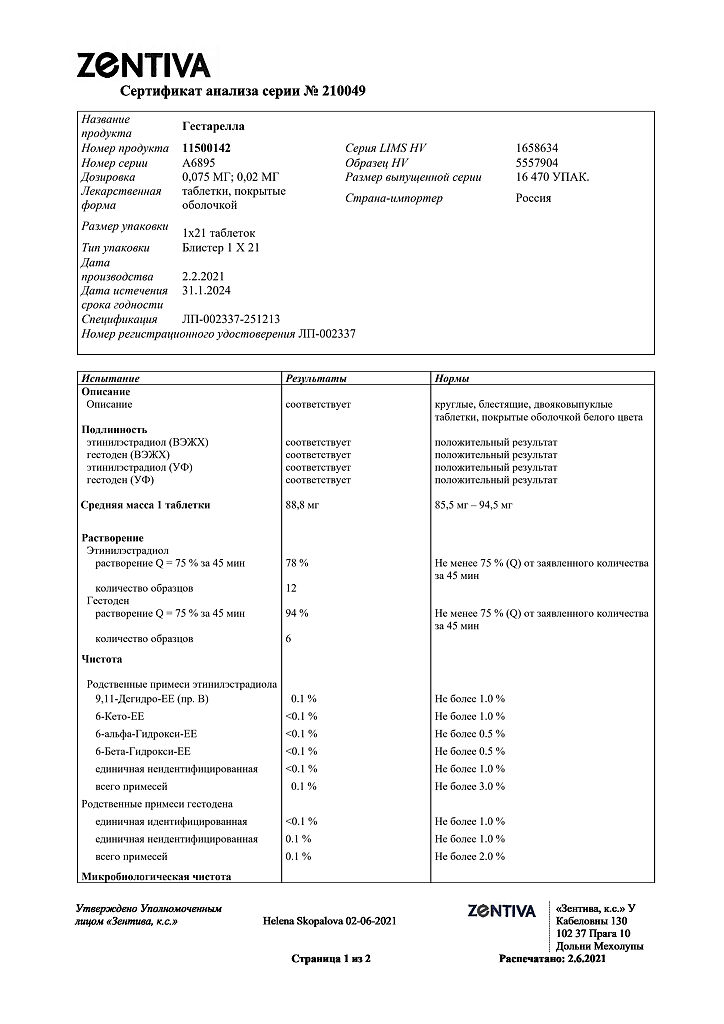
Each coated tablet contains:
Hestoden – 0.075 mg
ethinylestradiol – 0.020 mg
Associates:
core:
Calcium edetate sodium – 0.14 mg, lactose monohydrate – 36.865 mg, corn starch – 16.0 mg, povidone 25 – 1.7 mg, magnesium stearate – 0.2 mg;
coating:
sucrose – 19.631 mg, povidone 90 F – 0.20 mg, macrogol 6000 – 2.18 mg, calcium carbonate – 8.697 mg, talc – 4.242 mg, mountain glycol wax – 0.05 mg.
How to take, the dosage
How to take, the dosage
Gestarella® is taken in the order given on the package at about the same time every day. The tablets are taken orally with a small amount of water. Over the course of 21 days, one tablet is taken daily at a time.
The pills from the next package should be started the next day after a 7-day break, during which menstrual-like bleeding occurs. Bleeding usually begins 2 to 3 days after taking the last pill and may not end until the pills from the new pack are taken.
How to start taking Gestarella®.
If you did not use hormonal contraception in the previous month.
The pills should be started on the first day of your natural menstrual cycle (that is, the first day of your period). You can also start taking the pills from day 2 to
day 5 of your menstrual cycle, but you must use a barrier method of contraception for the first 7 days of the first cycle.
Transition from another combined oral contraceptive, vaginal ring, or transdermal patch.
Gestarella® is best started the day after taking the last active pill (the last pill containing the active ingredient) of the previous contraceptive, but never later than the day after the usual 7-day break in pills (for products with 21 pills per package) or after taking a placebo pill of the previous contraceptive (for products with 28 pills per package). In the case of the transdermal patch, the woman must begin taking the medication the day after it is removed, but never later than the day a new ring or patch is to be inserted.
Transition from progestagen-only contraceptives (“mini-pills”, injectables, implant) or a progestagen-releasing intrauterine system (IUD).
The switch from the mini-pill is possible at any time, from the implant or IUD on the day it is removed, from the injectables on the day the next injection is due, but in all these cases an additional barrier method of contraception must be used during the first 7 days of taking Gestarella®.
Use after an abortion in the first trimester of pregnancy.
The woman may begin taking the drug immediately. In this case the use of additional contraceptive methods is not required.
Use after childbirth or abortion in the second trimester of pregnancy.
The drug is started after childbirth, in the absence of breastfeeding, or an abortion in the second trimester of pregnancy. Gestarella® should be started between the 21st and 28th days after childbirth or during a second trimester abortion. If the woman starts taking it later, it is necessary to use an additional barrier method of contraception during the first 7 days of taking the pills. However, if sexual intercourse occurred before starting the drug, pregnancy must be ruled out before starting the contraceptive, or the woman must wait until her first menstrual bleeding.
The first menstrual cycle is usually prolonged by 1 week after the end of taking the drug. If a normal cycle is not restored within 2-3 months, a doctor should be consulted.
Taking missed pills.
If you are less than 12 hours late in taking the pill, contraceptive protection is not compromised. The pill should be taken immediately, as soon as the woman remembers the missed pill, the next pill should be taken at the usual time.
If the pill is taken more than 12 hours late, contraceptive protection may be reduced. If this happens, the following basic rules should be followed and appropriate action should be taken:
1. The pill should never be interrupted for more than 7 days.
2. A 7-day period of continuous pill taking is required to achieve the necessary suppression of the hypothalamic-pituitary-ovarian system.
In accordance with the above rules, the following is recommended.
The first week of taking the drug.
The woman should take the last missed pill as soon as she remembers it, even if it means taking two pills at once. After that, the pills should be taken at the usual time. Additionally, a barrier method of contraception, such as a condom, should be used for the next 7 days. If the woman was sexually active in the previous 7 days, it is necessary to consider the possibility of pregnancy. The greater the number of missed pills and the closer the skip is to a break in taking the drug, the higher the risk of pregnancy.
The second week of taking the drug.
The woman should take the last missed pill as soon as she remembers it, even if it means taking two pills at once. After that, the pills should be taken at regular times. If the pills were taken regularly for 7 days before the first missed pill, no additional contraceptive measures are needed. If the woman took the pills irregularly or missed more than one pill, additional contraceptive measures are needed for 7 days.
The third week of taking the drug.
There is a high risk of decreased contraceptive reliability because of the upcoming pill interruption. Even so, by adjusting your medication regimen, a decrease in contraceptive protection can be prevented. If either of the following two instructions is followed, there is no need for other contraceptive measures, provided that the woman has taken the pills correctly for 7 days before the first missed pill. Otherwise, the woman must adhere to the first recommendation and take other additional contraceptive measures within 7 days.
The woman should take the last missed pill as soon as she remembers it, even if it means taking two pills at once. After that, the pills should be taken at the usual time. The woman should start taking the next package immediately after she has finished taking the pills from the previous package. In this way, there will be no break between the pills. It is unlikely that you will have a bleeding discontinuation until the second package is complete, but you may have a small spot or a heavy vaginal bleeding.
Women may also stop taking pills from their current dose. If this happens, take a 7-day break, including the days she missed, then begin a new pill pack.
If a woman has forgotten to take her pills and she does not have withdrawal bleeding when she is not taking her pills, pregnancy should be excluded.
In case of gastrointestinal disturbances
If a woman has had vomiting or diarrhea within 4 hours of taking the pills, absorption may not be complete and additional contraceptive measures must be taken. In these cases, the recommendations for skipping the pill should be followed.
Changing the start day of the menstrual cycle
If a woman wants to delay the start day of her menstrual bleeding, she should start taking Gestarella® from the next package without interruption. It is possible to continue taking the new pack for as long as the woman wishes (until the pills in the pack are finished). During this period, the woman may have a “breakthrough” bleeding or a period of spotting. After a 7-day break, the woman must resume taking the medicine regularly.
If a woman wants to postpone the start of bleeding to another day of the week, she should shorten her immediate break from taking the pills by as many days as she wants. The shorter the interval, the greater the risk of “withdrawal” bleeding and “breakthrough” bleeding and smeary discharge during the second package (as well as if you postpone the start of bleeding).
Interaction
Interaction
The interaction between OCs and other medications may lead to the development of acyclic bleeding and/or contraceptive failure.
Long-term use of drugs inducers of microsomal liver enzymes, as a result of which clearance of sex hormones increases, may cause “breakthrough” bleeding and/or decrease contraceptive effectiveness of Gestarella®. This includes phenytoin, barbiturates, primidone, carbamazepine, rifampicin, oxcarbazepine, topiramate, felbamate, griseofulvin and preparations containing St. John’s wort.
It can also potentially enhance hepatic metabolism of the sex hormone HIV proteases (e.g., ritonavir) and non-nucleoside reverse transcriptase inhibitors (e.g., nevirapine) and combinations thereof.
When coadministering Gestarella® with perampanel, vemurofenib, bosentan, modafinil, or rufinamide, patients should consider the potential for decreased contraceptive efficacy due to accelerated sex steroid metabolism. It is recommended to use additional contraceptive methods (intrauterine devices or condoms) during the entire course of concomitant use of the drugs and for 2-6 months after discontinuation. If Gestarella® is used concomitantly with drugs inducible of microsomal liver enzymes and for 28 days after their withdrawal, additional use of barrier methods of contraception is required.
The use of etoricoxib in 120 mg dose together with OCs containing ethinylestradiol increases AUC0-24 of ethinylestradiol by 50-60 %. This increase in ethinylestradiol concentration should be taken into account when choosing an appropriate OC for coadministration with etoricoxib. This fact may lead to an increased incidence of thromboembolism due to increased ethinylestradiol exposure.
A decrease in effective plasma concentration of ethinylestradiol is observed during concomitant administration of some antibiotics (penicillins, tetracycline) due to changes in the microflora in the gut, so during antibiotic therapy (except rifampicin and griseofulvin) and within 7 days after their withdrawal an additional barrier method of contraception should be used.
Non-steroidal anti-inflammatory drugs (NSAIDs) decrease the effectiveness of Gestarella®.
The combined oral contraceptive drugs may affect the metabolism of other drugs, so their plasma and tissue concentrations may increase (e.g., cyclosporine) or decrease (e.g., lamotrigine).
Special Instructions
Special Instructions
If any of the conditions or risk factors listed below are present, the use of Gestarella® should be discussed with the woman in advance. If any of the conditions or risk factors worsen or appear for the first time, women should be advised to see their healthcare provider to decide whether to continue or stop taking the drug.
With regard to the fact that the contraceptive effect of Gestarella® is mostly seen by the 14th day after initiation of use, it is recommended that additional non-hormonal (barrier) contraceptive methods be used in the first 2 weeks of taking the drug.
Cardiovascular disease
Risk of venous thromboembolism (VTE)
The use of any OCs increases the risk of VTE compared to women not taking these drugs.
Before starting Gestarella® , a woman should discuss the nearly twofold higher risk of VTE compared to other OCs containing levonorgestrel, noregestimate, or norethisterone. This risk is highest in the first year of use or when resuming use after a break of 4 weeks or more.
The results of an epidemiologic study showed that among women who are not taking OCs and are not pregnant, VTE occurs in about 2 in 10,000 within a year. However, some women may have a much higher risk depending on the underlying risk factors they have (see below). It is estimated that 9 to 12 out of 10,000 women taking OCs containing gestoden will experience VTE within a year. In both cases, the number of VTEs per year is less than the number of expected VTEs during pregnancy or in the postpartum period.
Risk factors for thromboembolic complications
The risk of venous and arterial thromboembolic complications in women taking OC may increase significantly if additional risk factors are present, especially if the risk factors are multiple (see Table #1 and #2).
Gestarella® is contraindicated if a woman has multiple risk factors for VTE or ATE. If a woman has more than one risk factor, the risk of thromboembolic complications may be greater than the simple sum of the individual factors. If the benefit-risk ratio is negative, Gestarella® should not be prescribed.
Risk factors for VTE
Table 1. Risk factors for VTE
Risk factor
Comment
Obese (body mass index (BMI) > 30 kg/m2)
The risk increases significantly with increasing BMI.
This risk factor is especially important in women with additional risk factors.
Long-term immobilization, extensive surgery, any lower extremity or pelvic surgery, neurosurgery, or extensive trauma
Note: Temporary immobilization, including air travel of ? 4 hours, may also be a risk factor for VTE, especially in women with other risk factors.
In these situations, it is recommended that the patches/pills/rings be discontinued (if elective surgery, at least four weeks before) and not resumed for 2 weeks after complete remobilization. Another method of contraception should be used to avoid unwanted pregnancy.
The prescription of antithrombotic therapy should be considered if Gestarella® has not been discontinued beforehand.
A strong family history (history of VTE in siblings or parents, especially at a relatively young age, e.g., younger than 50 years)
If a hereditary predisposition is suspected, women should be referred for a specialist consultation before deciding to use any OCs.
Other medical conditions associated with the development of VTE
Cancer, systemic lupus erythematosus, hemolytic-uremic syndrome, chronic inflammatory bowel disease (Crohn’s disease or ulcerative colitis), and sickle cell anemia.
Age
Particularly over the age of 35.
There is no consensus on the possible role of varicose veins and superficial venous thrombophlebitis in the development or progression of venous thrombosis.
The increased risk of thromboembolism during the first 6 weeks of the postpartum period should be considered.
The symptoms of VTE (deep vein thrombosis (DVT) and pulmonary artery thromboembolism (PATE))
If a woman has symptoms of VTE, she should seek immediate emergency medical attention and inform her doctor that she is taking OCs.
The symptoms of VTE may include:
unilateral swelling of the lower extremity and/or foot or along the vein in the lower extremity;
pain in the lower extremities or painfulness when touched, which may be felt only when standing or walking;
increased skin temperature of the affected lower extremity;
redness or pallor of the skin of the lower extremity.
The symptoms of TELA may include:
sudden unexplained shortness of breath or rapid breathing;
sudden cough that may be accompanied by hemoptysis;
sharp chest pain;
severe dizziness;
frequent or irregular heartbeat.
Some of these symptoms (such as “shortness of breath” or “cough”) are nonspecific and can be misunderstood as manifestations of more frequent and less severe conditions (such as respiratory infections).
Other signs of vascular occlusion may include: sudden pain, swelling, and mild cyanosis of the limb.
In ocular vascular occlusion, symptoms can range from painless blurred vision, which may progress, to vision loss. Sometimes vision loss can be acute.
Risk of arterial thromboembolism (ATE)
Epidemiological studies have linked OC use to an increased risk of ATE (myocardial infarction) or cerebrovascular disease (e.g., transient ischemic attack (TIA), stroke). Arterial thromboembolic events can be fatal.
Risk factors for ATEs
Table 2. Risk Factors for ATE
Risk Factor
Commentary
Age
Especially over the age of 35.
Smoking
A woman should be advised not to smoke if she wants to take OCs. Women over 35 who continue to smoke should be strongly advised to use another method of contraception.
Arterial hypertension
–
Obesity (BMI > 30 kg/m2)
The risk increases significantly with increasing BMI. This risk factor is especially important in women with additional risk factors.
A strong family history (arterial thromboembolism in siblings or parents, especially at a relatively young age, such as under 50)
If a hereditary predisposition is suspected, women should be referred for a specialist consultation before deciding to use any OCs.
Migraine
An increase in the frequency or severity of migraines while taking OCs (which may be a precursor condition to the development of cerebrovascular disease) may be grounds for immediate discontinuation of the drug.
Other medical conditions associated with adverse vascular events
Diabetes mellitus, hyperhomocysteinemia, coronary heart disease and atrial fibrillation, dyslipoproteinemia, and systemic lupus erythematosus.
Symptoms of ATE
If a woman has symptoms of ATE, she should seek immediate emergency medical attention and inform her doctor that she is taking OCs.
The symptoms of cerebrovascular disease may include:
sudden numbness or weakness of muscles in the face, arm, or leg, especially one side of the body;
sudden difficulty moving, dizziness, loss of balance or coordination;
sudden confusion, trouble speaking or understanding;
sudden, severe, or prolonged headache for no particular reason;
consciousness or fainting, with or without seizures.
Transient symptoms indicate the development of an event called a transient ischemic attack (TIA).
Symptoms of myocardial infarction (MI) may include:
Pain, discomfort, pressure, heaviness, feeling of tightness or distension in the chest or behind the sternum, with irradiation to the back, neck, jaw, upper extremity, epigastrium area;
Feeling overfilled in the stomach, upsetting digestion, or choking;
sweating, nausea, vomiting, and dizziness;
extreme weakness, restlessness, or shortness of breath;
frequent or irregular heartbeat.
There is evidence of an increased incidence of venous and arterial thrombosis and thromboembolism when taking OCs.
However, the incidence of venous thromboembolism (VTE) that develops while taking OC is lower than the incidence of VTE that occurs during pregnancy (6 per 10,000 pregnant women per year).
In women taking OC, very rare cases of thrombosis of other blood vessels, such as hepatic, mesenteric, renal arteries and veins, and central retinal vein and its branches, have been described. An association with OC intake has not been proven.
Tumors
One significant risk factor for cervical cancer is persistent papillomavirus infection. There have been reports of an increased risk of cervical cancer with long-term use of OCs. However, the association with OC use has not been proven. This increase may be associated with the detection of cervical abnormalities during the compulsory examination prior to the prescription of pills or the characteristics of sexual behavior (less frequent use of barrier methods of contraception).
It has also been found that there is a slightly increased relative risk of breast cancer diagnosed in women who have used OCs, but the association has not been proven. The increased risk observed may be due not only to an earlier diagnosis of breast cancer in women who use OC, but also to the biological effects of sex hormones or a combination of the two. Breast cancer in women who are taking or have taken OCs in the past is usually detected at clinically less advanced stages than in women who have never taken the drugs.
In rare cases, the development of liver tumors has been observed with the use of OCs. This should be considered when making a differential diagnosis if severe abdominal pain, liver enlargement, or signs of intra-abdominal bleeding occur.
Contraindications
Contraindications
Gestarella® is contraindicated in the presence of any of the conditions/diseases listed below. If any of the listed conditions develops for the first time while taking it, the drug should be stopped immediately.
Thromboses (venous and arterial) and thromboemboli, current or past history (including deep vein thrombosis, pulmonary embolism, myocardial infarction);
Conditions preceding thrombosis (including angina pectoris);
Cerebrovascular disease: Stroke, current and history of transient ischemic attacks;
Multiple or significant risk factors for venous or arterial thrombosis, including complicated cardiac valve lesions, atrial fibrillation, cerebral vascular disease or coronary artery disease, severe dyslipoproteinemia uncontrolled arterial hypertension, major surgery, prolonged immobilization, air travel longer than 4 hours, lower extremity and pelvic surgery, neurosurgery, and smoking over the age of 35;
Congenital or acquired predisposition to arterial or venous thrombosis (resistance to activated protein C (including factor V Leiden), antithrombin III deficiency, protein C deficiency, protein S deficiency, hyperhomocysteinemia, presence of antibodies to phospholipids (anticardiolipin, lupus anticoagulant);
Migraine with focal neurological symptoms currently or in the history;
Diabetes mellitus with vascular complications;
Pancreatitis with significant hypertriglyceridemia, current or history;
Liver failure and severe liver disease, including Dubin-Johnson and Rotor syndromes (until normalization of liver enzymes);
Liver tumors (benign or malignant), current or history;
Identified or suspected hormone-dependent malignancies (including genital or breast malignancies);
Childhood and adolescence (before menarche);
Vaginal bleeding of unclear genesis;
Pregnancy or suspected pregnancy;
Breastfeeding period;
Hypersensitivity to any of the ingredients of Gestarella®;
Obesity (body mass index greater than 30 kg/m2);
Extensive trauma;
Galactose intolerance, lactase deficiency or glucose-galactose malabsorption, fructose intolerance or sucrose-isomaltase deficiency (the drug contains lactose monohydrate and sucrose).
WARNING
The potential risks and expected benefits of combined oral contraceptives (OCs) should be carefully weighed in each individual case with the following diseases/conditions and risk factors:
Risk factors for thrombosis and thromboembolism: Smoking, obesity (body mass index less than 30 kg/m2), dyslipoproteinemia, arterial hypertension, air travel longer than 4 hours, migraine without focal neurological symptoms, uncomplicated heart valve defects, hereditary predisposition to thrombosis (thrombosis, myocardial infarction or stroke in young age in any of the immediate family members);
Other conditions in which peripheral circulatory disorders may be noted: diabetes mellitus, cancer, systemic lupus erythematosus, hemolytic-uremic syndrome, Crohn’s disease and ulcerative colitis, sickle cell anemia, superficial vein phlebitis;
Hereditary angioedema;
Hypertriglyceridemia;
Illnesses that first occurred or worsened during pregnancy or on previous use of sex hormones (e.g., jaundice, cholestasis, gallbladder disease, otosclerosis with hearing impairment, porphyria, herpes during pregnancy, Sidengam’s chorea);
Depression;
Epilepsy;
Postpartum.
Side effects
Side effects
The most frequently reported adverse reactions (ARs) in clinical trials and post-marketing observations with OCs are bleeding/bleeding. Women taking OCs had an increased risk of arterial and venous thrombotic and thromboembolic events, including myocardial infarction, stroke, transient ischemic attack, venous thrombosis and pulmonary embolism.
The adverse reactions are divided into groups according to MedDRA (Medical Dictionary of Regulatory Activity Terminology) terminology. The frequency of side effects was determined according to the World Health Organization classification: very common â¥10%; common â¥1% and < 10%; infrequent â¥0.1% and < 1%; rare â¥0.01% and < 0.1%; very rare < 0.01%; unknown frequency (the frequency of side effects could not be determined from available data).
Immune system disorders: rare – hypersensitivity reactions; unknown frequency – exacerbation of systemic lupus erythematosus.
Disorders of metabolism and nutrition: often – weight gain, fluid retention; rarely – weight loss; unknown frequency – exacerbation of porphyria.
Mental disorders: frequent – depressed mood, mood swings; infrequent – decreased libido; rare – increased libido; unknown frequency – nervousness, depression.
Nervous system disorders: frequent – headache, migraine; unknown frequency – dizziness, worsening chorea.
Visual system disorders: rare – contact lens intolerance (discomfort when wearing contact lenses); unknown frequency – optic neuritis.
Vascular disorders: rare – venous thromboembolism, arterial thromboembolism.
Gastrointestinal disorders: frequent – nausea, vomiting; infrequent – abdominal pain, diarrhea; very rare – pancreatitis; unknown frequency – bloating, colitis.
Liver and biliary tract disorders: rarely – cholelithiasis.
Skin and subcutaneous tissue disorders: infrequent – skin rash, including uretic; rare – erythema nodosum, erythema multiforme; unknown frequency – acne, hirsutism, alopecia.
Gender and breast disorders: often – feeling of tension, painful breasts, vaginal discharge; infrequent – enlargement of the breasts; rarely – discharge from the breasts; unknown frequency – painful menstrual-like bleeding, vaginitis, candidiasis vulvovaginitis, pain in the breasts.
Laboratory and instrumental data: infrequent – hyperlipidemia.
The following serious adverse events have been reported in women using OCs. Additional information on possible side effects is presented in the section “Special indications”:
Venous thromboembolic disorders;
Arterial thromboembolic disorders;
Cerebrovascular disorders;
Elevated blood pressure;
Hypertriglyceridemia;
Decreased glucose tolerance or effect on peripheral insulin resistance;
Liver tumors (benign and malignant);
Disordered liver function;
Chloasma.
The onset or worsening of conditions for which there is no proven association with OC use: Jaundice and/or pruritus associated with cholestasis; gallstones formation; hemolytic-uremic syndrome; herpes of pregnancy; hearing loss associated with otosclerosis; Crohn’s disease; ulcerative colitis; cervical cancer (see See section “Special Indications”).
Overdose
Overdose
There are no available data on the development of serious adverse reactions in overdose. The following symptoms may be observed: nausea, vomiting, slight bleeding from the vagina in young girls.
Treatment: symptomatic therapy is carried out. There is no specific antidote.
Similarities
Similarities
Additional information
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 ° C. Keep out of reach of children! |
| Manufacturer | Haupt Pharma Münster GmbH, Germany |
| Medication form | pills |
| Brand | Haupt Pharma Münster GmbH |
Other forms…
Related products
Gynecology and Obstetrics
Gynecology and Obstetrics
Prepidil, intracervical gel 0.5 mg/3 g syringes with catheter
Buy Gestarella, 75 mcg+20 mcg 21 pcs with delivery to USA, UK, Europe and over 120 other countries.