No products in the cart.
Description
Pharmacological group: immunomodulatory agents, interferons.
ATC code – L03AB05
Pharmacological properties
Immunobiological properties
Henferon®
Immunobiological properties. GENFERON® is a combined preparation the action of which is conditioned by its constituent components. It has both local and systemic action.
The preparation Genferon contains recombinant human interferon
alpha-2b produced by Escherichia coli strain which has been genetically engineered with human interferon alpha-2b gene.
Interferon alpha-2b has antiviral, immunomodulatory, antiproliferative and antibacterial action. The antiviral effect is mediated by the activation of a number of intracellular enzymes that inhibit viral replication. Immunomodulatory action is first of all manifested by strengthening of cell-mediated reactions of immune system which increases the effectiveness of immune response against viruses, intracellular parasites and cells which have undergone tumor transformation.
This is achieved through activation of CD8+ T-helpers, NK-cells (natural killer cells), increasing differentiation of В-lymphocytes and their production of antibodies, activation of monocytic-macrophage system and phagocytosis as well as increasing the expression of histocompatibility complex type I molecules, which increases the recognition of infected cells by the immune system.
The activation under the influence of interferon of leukocytes contained in all layers of the mucous membrane provides their active participation in the liquidation of pathological foci; besides, due to the influence of interferon the restoration of the production of secretory immunoglobulin A is achieved. Antibacterial effect is mediated by reactions of the immune system enhanced under the influence of interferon.
Taurine promotes normalization of metabolic processes and tissue regeneration, has a membrane stabilizing and immunomodulatory action. As a strong antioxidant, taurine directly interacts with reactive oxygen species, the excessive accumulation of which contributes to pathological processes.
Taurine helps to preserve the biological activity of interferon, increasing the therapeutic effect of the drug.
Benzocaine (anesthesin) is a local anesthetic. It reduces cell membrane permeability to sodium ions, displaces calcium ions from the receptors located on the inner surface of the membrane, blocks conduction of nerve impulses. Prevents the occurrence of pain impulses in the endings of sensitive nerves and their conduction along the nerve fibers.
It has only local effect without being absorbed into the systemic blood flow.
Pharmacokinetics
In rectal administration of the drug a high bioavailability (more than 80%) of interferon is observed, due to which both local and expressed systemic immunomodulatory effects are achieved; At intravaginal application due to high concentration in the focus of infection and fixation on the cells of the mucous membrane a pronounced local antiviral, antiproliferative and antibacterial effect is achieved, while the systemic effect is insignificant due to the low absorption capacity of the vaginal mucosa.
The maximum concentration of interferon in blood serum is achieved 5 hours after administration of the drug. The main way of excretion of α-interferon is renal catabolism. Half-life period is 12 hours, which determines the necessity of using the drug 2 times
a day.
Indications
Indications
As part of complex therapy for infectious and inflammatory diseases of the urogenital tract in adults: genital herpes, chlamydia, ureaplasmosis, mycoplasmosis, recurrent vaginal candidiasis, gardnerellosis, trichomoniasis, human papillomavirus infection, bacterial vaginosis, cervical erosion, cervicitis, vulvovaginitis, bartholinitis, adnexitis, prostatitis, urethritis, balanitis, balanoposthitis.
As part of complex therapy of acute bronchitis in adults.
As part of complex therapy for chronic recurrent cystitis of bacterial etiology in adults.
As part of complex therapy for chronic endometritis in adults.
Pharmacological effect
Pharmacological effect
Pharmacological group: immunomodulatory agents, interferons.
ATX code – L03AB05
PHARMACOLOGICAL PROPERTIES
Immunobiological properties
GENFERON® is a combination drug, the effect of which is determined by the components included in its composition. Has local and systemic effects.
The drug Genferon® contains recombinant human interferon
alpha-2b, produced by a strain of the bacterium Escherichia coli, into which the human interferon alpha-2b gene has been introduced using genetic engineering.
Interferon alpha-2b has antiviral, immunomodulatory, antiproliferative and antibacterial effects. The antiviral effect is mediated by the activation of a number of intracellular enzymes that inhibit viral replication. The immunomodulatory effect is manifested, first of all, by enhancing cell-mediated reactions of the immune system, which increases the effectiveness of the immune response against viruses, intracellular parasites and cells that have undergone tumor transformation.
This is achieved through the activation of CD8+ T killer cells, NK cells (natural killer cells), increased differentiation of B lymphocytes and their production of antibodies, activation of the monocyte-macrophage system and phagocytosis, as well as increased expression of molecules of the major histocompatibility complex type I, which increases the likelihood of recognition of infected cells by cells of the immune system.
Activation under the influence of interferon of leukocytes contained in all layers of the mucous membrane ensures their active participation in the elimination of pathological foci; in addition, due to the influence of interferon, restoration of the production of secretory immunoglobulin A is achieved. The antibacterial effect is mediated by reactions of the immune system, enhanced under the influence of interferon.
Taurine helps normalize metabolic processes and tissue regeneration, has membrane-stabilizing and immunomodulatory effects. Being a strong antioxidant, taurine directly interacts with reactive oxygen species, the excessive accumulation of which contributes to the development of pathological processes.
Taurine helps maintain the biological activity of interferon, enhancing the therapeutic effect of the drug.
Benzocaine (anesthetic) is a local anesthetic. Reduces the permeability of the cell membrane to sodium ions, displaces calcium ions from receptors located on the inner surface of the membrane, and blocks the conduction of nerve impulses. Prevents the occurrence of pain impulses at the endings of sensory nerves and their conduction along nerve fibers.
It has an exclusively local effect, without being absorbed into the systemic circulation.
Pharmacokinetics
With rectal administration of the drug, high bioavailability (more than 80%) of interferon is observed, and therefore both local and pronounced systemic immunomodulatory effects are achieved; when used intravaginally, due to the high concentration at the site of infection and fixation on the cells of the mucous membrane, a pronounced local antiviral, antiproliferative and antibacterial effect is achieved, while the systemic effect is insignificant due to the low absorption capacity of the vaginal mucosa.
The maximum concentration of interferon in the blood serum is achieved 5 hours after administration of the drug. The main route of excretion of α-interferon is renal catabolism. The half-life is 12 hours, which necessitates the use of the drug 2 times
per day.
Special instructions
Special instructions
To prevent urogenital reinfection, it is recommended to consider simultaneous treatment of the sexual partner.
The drug can be used during menstruation.
The drug GENFERON®
does not affect the performance of potentially hazardous activities that require special attention and quick reactions (driving vehicles, machinery, etc.).
Active ingredient
Active ingredient
Benzocaine, Interferon alpha-2b, Taurine
Composition
Composition
Active ingredients:
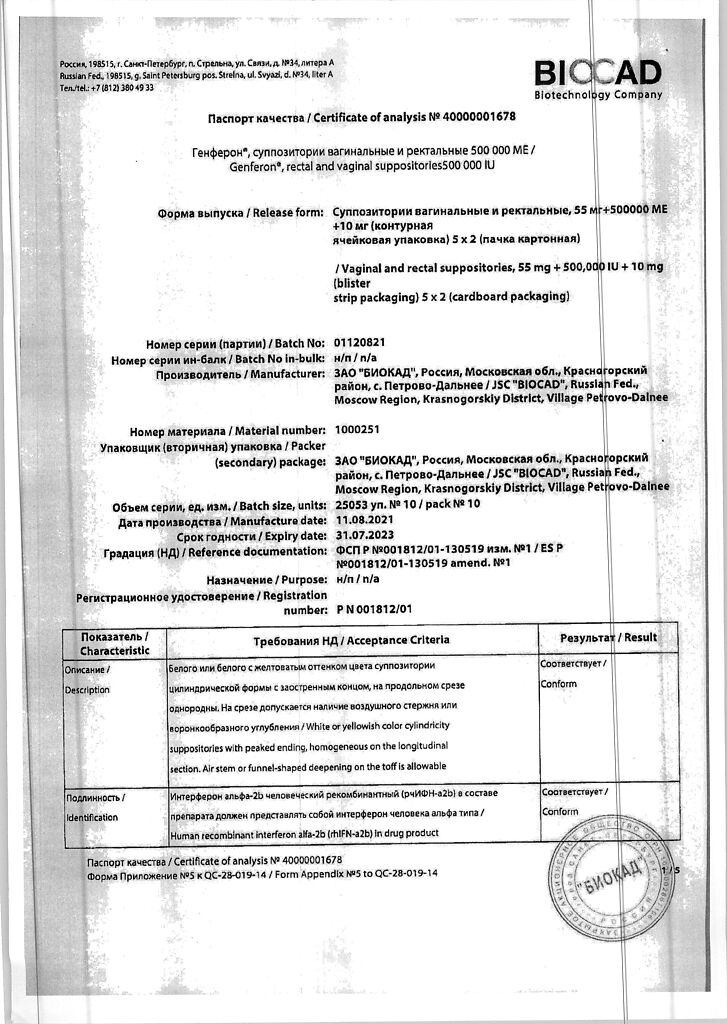
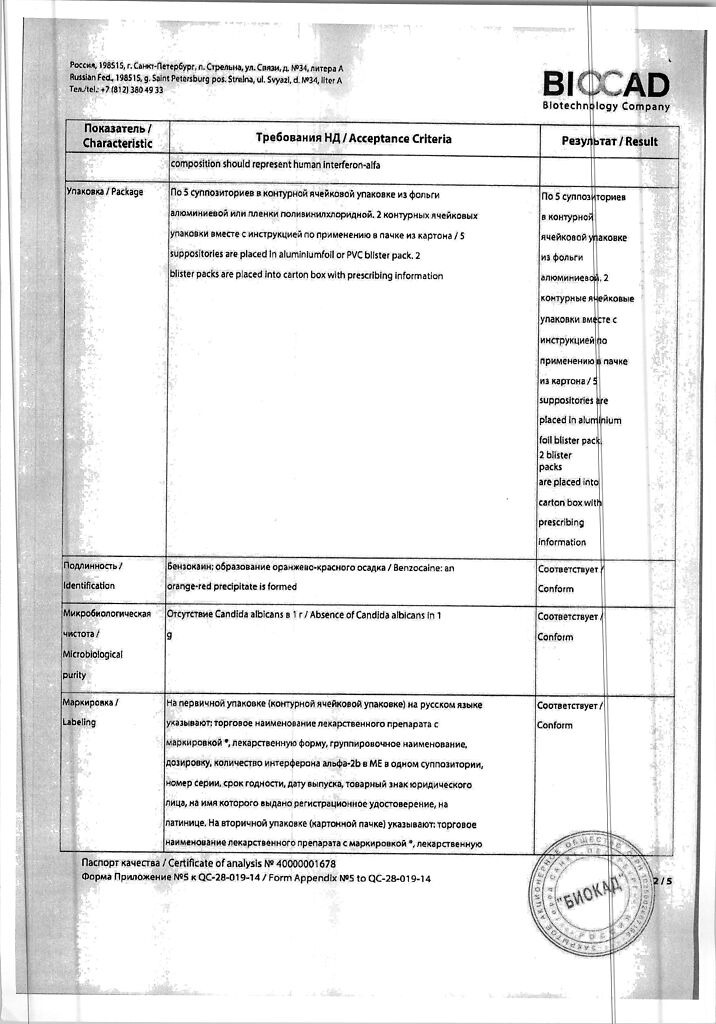
human recombinant interferon alpha-2b (rhIFN-α-2b); taurine0.01 g; benzocaine 0.055 g;
Excipients:
solid fat – q.s. until a suppository weighing 1.65 g is obtained; dextran 60000 – 0.0015 g; macrogol 1500 – 0.124 g; polysorbate 80 – 0.033 g; emulsifier T2 – 0.132 g; sodium hydrogen citrate – 0.0001 g; citric acid – 0.0015 g; purified water – 0.066 g
Pregnancy
Pregnancy
Indicated use for normalization of local immunity during pregnancy 13-40 weeks as part of complex therapy of genital herpes, chlamydia, ureaplasmosis, mycoplasmosis, cytomegalovirus infection, human papillomavirus infection, bacterial vaginosis in the presence of itching, discomfort and pain in the lower parts of the urogenital tract.
Clinical studies have proven the safety of intravaginal use of the drug Genferon® 250,000 IU during pregnancy 13-40 weeks. The safety of using the drug in the first trimester of pregnancy has not been studied.
Contraindications
Contraindications
Individual intolerance to interferon and other substances included in the drug.
With caution: exacerbation of allergic and autoimmune diseases.
Side Effects
Side Effects
The drug is well tolerated by patients.
Very rare adverse reactions (< 1/10000):
General and administration site disorders
Allergic reactions, including local ones, are possible. Continuation of treatment is possible after consultation with a doctor.
Phenomena that occur with the use of all types of interferon alpha-2b, such as chills, fever, fatigue, loss of appetite, muscle and headaches, joint pain, sweating, as well as leukemia and thrombocytopenia, may be observed, but more often they occur when the daily dose is exceeded by more than
10,000,000 IU. To date, no serious side effects have been observed.
As with any other interferon alfa-2b drug, if the temperature rises after its administration, a single dose of paracetamol is possible
500 – 1000 mg.
Interaction
Interaction
Genferon® is most effective in combination with drugs (including antibiotics and other antimicrobial drugs) used to treat urogenital diseases.
Non-narcotic analgesics and anticholinesterase drugs enhance the effect of benzocaine.
Benzocaine reduces the antibacterial activity of sulfonamides.
Overdose
Overdose
No cases of overdose with GENFERON® have been reported. At
accidental simultaneous administration of more suppositories than was intended
prescribed by a doctor, further administration should be suspended for 24 hours, after
then treatment can be resumed according to the prescribed regimen.
Storage conditions
Storage conditions
At 2–8 °C
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Biocad, Russia
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | At 2-8 °C |
| Manufacturer | Biocad, Russia |
| Medication form | Vaginal and rectal suppositories |
| Brand | Biocad |
Other forms…
Related products
Buy Genferon, vaginal and rectal suppositories 55 mg+500000 me+10 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.