No products in the cart.
Description
Pharmacotherapeutic group
Immunomodulatory agent.
ATC code:L03A
Pharmacological properties
Pharmacodynamics.The drug Genferon® Lite, nasal drops, has antiviral, immunomodulatory, anti-inflammatory, antiproliferative, antibacterial action, has local regenerating, membranostabilizing and antioxidant properties.
Interferon alpha blocks viral reproduction at the stage of synthesis of specific proteins and prevents infection of uninfected cells of the nasal cavity mucosa which is the site of invasion of pathogens and the primary focus of inflammation in respiratory infections.
The immunomodulatory action is manifested by strengthening the cell-mediated reactions of the immune system, which increases the efficiency of the immune response against foreign agents. This is achieved by activation of CD8+ T-cells, NK-cells (natural killer cells) and increasing differentiation.
B-lymphocytes and their production of antibodies, activation of monocytic-macrophage system and phagocytosis, as well as increasing the expression of histocompatibility complex type I molecules, which increases the recognition of infected cells by cells of the immune system.
The activation under the influence of interferon of leukocytes contained in all layers of the mucous membrane provides their active participation in the liquidation of pathological foci; besides, due to the influence of interferon, the restoration of the production of secretory immunoglobulin A is achieved. Antimicrobial effect is mediated by reactions of the immune system enhanced under the influence of interferon.
Taurine contained in the drug normalizes metabolic processes in tissues, promotes regeneration and more rapid recovery of the nasal cavity mucosa damaged by pathological process.
Pharmacokinetics.
In intranasal application Genferon ® Light nasal drops produce high concentration of interferon in the infection nidus and produce significant local antiviral and immunostimulating effect.
Systemic absorption of the drug is insignificant. During intranasal administration human recombinant interferon alpha-2b is detected in small amounts in lung tissue and blood. In the body, biotransformation occurs mainly in the kidneys with a half-life (T½) of 5.1 h. A small amount of the drug entering the systemic bloodstream has a systemic immunomodulatory effect.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
1 ml of the solution contains:
Active ingredients: interferon alpha-2b human recombinant* 10,000 IU, taurine 0.80 mg;
excipients: disodium edetatadihydrate – 0.02 mg, glycerol – 7.00 mg, dextran-35-45 thousand. – 2.4 mg, polysorbate-80 – 1.0 mg, sodium chloride – 0.8 mg, potassium chloride – 0.02 mg, sodium hydrophosphate – 0.115 mg, potassium dihydrophosphate – 0.02 mg, water for injection – up to 1 ml.
How to take, the dosage
How to take, the dosage
In the first signs of illness, Genferon®light is put into the nose for 5 days.
In children from 29 days to 11 months, 29 days, 1 drop in each nasal passage 5 times a day (single dose 1,000 IU, daily dose 5,000 IU).
In children 1 to 3 years of age, 2 drops in each nasal passage 3 to 4 times daily (single dose 2,000 IU, daily dose 6,000 to 8,000 IU).
In children 3 to 14 years of age, 2 drops in each nasal passage 4 to 5 times daily (single dose of 2,000 ME, daily dose of 8,000 to 10,000 ME).
Interaction
Interaction
Special Instructions
Special Instructions
Contraindications
Contraindications
Hypersensitivity to interferon alfa-2b or other drug components.
Side effects
Side effects
Local allergic reactions (burning sensation, itching) are possible. These phenomena are reversible and disappear on their own within 72 hours after discontinuation of the drug. Treatment may be continued only after consulting a physician.
Overdose
Overdose
Pregnancy use
Pregnancy use
Additional information
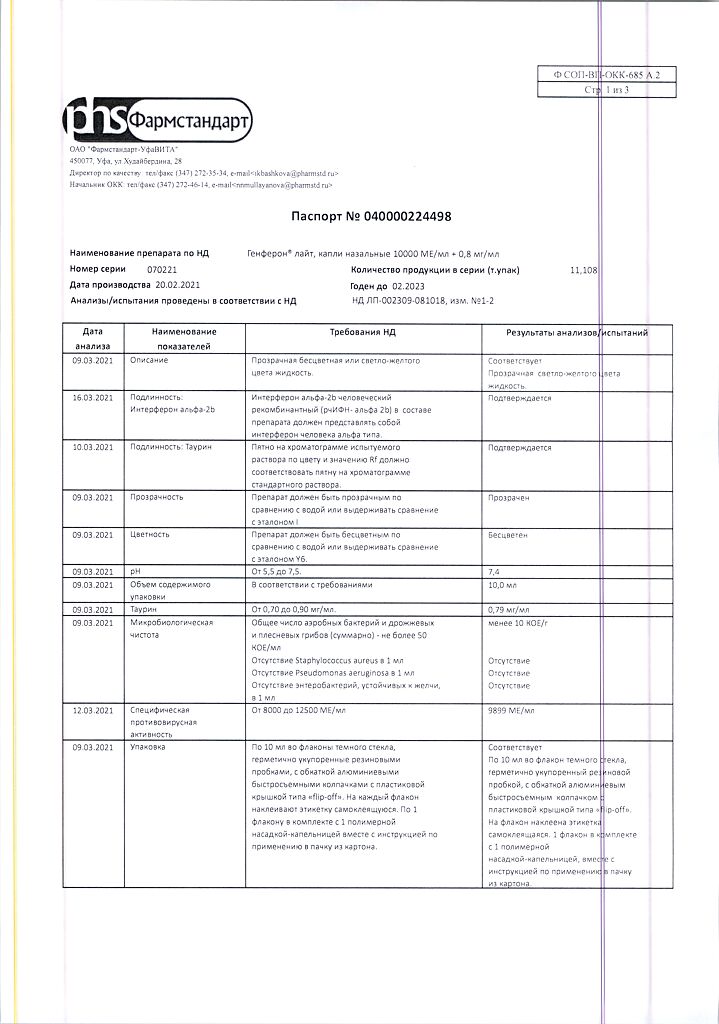
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature of 2 to 8 ° C. |
| Manufacturer | Pharmstandard-UfaVITA, Russia |
| Medication form | nasal drops |
| Brand | Pharmstandard-UfaVITA |
Other forms…
Related products
Buy Genferon Light, drops 10000 me/ml+0.8 mg/ml 10 ml with delivery to USA, UK, Europe and over 120 other countries.